Q3C GUIDELINE FOR RESIDUAL SOLVENTS
- 2. Contents • Introduction • Scope of the Guideline • Classification • Limits of Residual Solvents • Options for Describing Limits of Class 2 Solvents • Analytical Procedures • Reporting Levels of residual solvents • Residual Solvents in Pharmaceuticals • Glossary
- 3. Introduction Residual solvents in Pharmaceuticals are defined in ICH Q3C as organic volatile chemicals that are used or produced in the manufacture of drug substances, excipients or in the preparation of drug products. They are not completely removed by practical manufacturing techniques. Residual solvents are used in manufacture either to enhance the yield or determine characteristics of the substances such as crystal form, purity and solubility.There is no therapeutic benefit from residual solvents. Since there is no therapeutic benefit from residual solvents, all residual solvents should be removed to the extent possible to meet product specifications, good manufacturing practices, or other quality-based requirements.
- 4. To recommend acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient.The guideline recommends use of less toxic solvents and describes levels considered to be toxicologically acceptable for some residual solvents. The guideline applies to all dosage forms and routes of administration. This guidelines does not address all possible solvents, only those identified in drugs at that time, neither address solvents intentionally used as excipients nor solvates. The maximum acceptable intake per day of residual solvent in pharmaceutical products is defined as “permitted daily exposure” (PDE) Previously, another terms were used like “Tolerable daily intake” (TDI) & “Acceptable daily intake” (ADI) by different organization & authorities, but now usually this new term “PDE” is used Scope of the Guideline
- 5. Classification Residual Solvents are classified according to their Risk Assessments to human health to 3 main classes: Class 1 solvents Solvents to be avoided Class 2 solvents Solvents to be limited Class 3 solvents Solvents with low toxic potential
- 6. ClassificationofResidualSolventsby RiskAssessment Class 1 solvents Solvents to be avoided Known human carcinogens, strongly suspected human carcinogens, and environmental hazards Class 2 solvents Solvents to be limited Non-genotoxic animal carcinogens or possible causative agents of other irreversible toxicity such as neurotoxicity or teratogenicity. Class 3 solvents Solvents with low toxic potential Solvents with low toxic potential to man; no health-based exposure limit is needed.
- 7. Limits of Residual Solvents
- 8. Class 1 Solvents Solvents to Be Avoided Solvents in Class 1 should not be employed in the manufacture of drug substances,excipients, and drug products because of their unacceptable toxicity or their deleterious environmental effect. However, if their use is unavoidable in order to produce a drug product with a significant therapeutic advance, then their levels should be restricted as shown inTable unless otherwise justified. Solvent Concentration limit (ppm) Concern Benzene 2 Carcinogen Carbon tetrachloride 4 Toxic and environmental hazard 1,2-Dichloroethane 5 Toxic 1,1-Dichloroethene 8 Toxic 1,1,1-Trichloroethane 1500 Environmental hazard
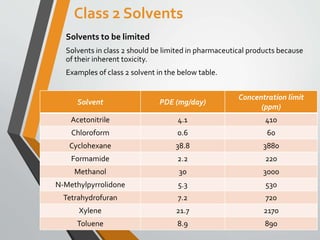
- 9. Class 2 Solvents Solvents to be limited Solvents in class 2 should be limited in pharmaceutical products because of their inherent toxicity. Examples of class 2 solvent in the below table. Solvent PDE (mg/day) Concentration limit (ppm) Acetonitrile 4.1 410 Chloroform 0.6 60 Cyclohexane 38.8 3880 Formamide 2.2 220 Methanol 30 3000 N-Methylpyrrolidone 5.3 530 Tetrahydrofuran 7.2 720 Xylene 21.7 2170 Toluene 8.9 890
- 10. Class 3 Solvents (Solvents with low toxic potential) •Solvents in Class 3 may be regarded as lower risk to human health. However, there are no long-term toxicity or carcinogenicity studies for many of the solvents in Class 3. •These solvents are considered of no human health hazard •Available data indicate that they are less toxic in acute or short-term studies and negative in genotoxicity studies. • It is considered that amounts of these residual solvents of 50 mg per day or less (corresponding to 5000 ppm or 0.5% under Option 1) would be acceptable without justification. • Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and GMP.
- 11. Class 3 Solvents (Continue) Acetone Methylisobutyl ketone Ethyl ether Acetic acid Heptane Dimethyl sulfoxide Ethyl formate Anisole Ethanol Formic acid Methyl acetate Ethyl acetate 3-Methyl-1-butanol Butyl acetate tert-Butylmethyl ether Isobutyl acetate 1-Butanol Methylethyl ketone 1-Pentanol 2-Methyl-1-propanol Heptane Isopropyl acetate 2-Butanol Pentane 1-Propanol Examples of Class 3 solvents which should be limited by GMP or other quality based requirements.
- 12. The following solvents may also be of interest to manufacturers of excipients, drug substances, or drug products. However, no adequate toxicological data on which to base a PDE was found. •Manufacturers should supply justification for residual levels of these solvents in pharmaceutical products. •Examples : 1,1-Diethoxypropane Methylisopropyl ketone 1,1-Dimethoxymethane Methyltetrahydrofuran 2,2-Dimethoxypropane Petroleum ether Isooctane Trichloroacetic acid Isopropyl ether Trifluoroacetic acid Solvents for which No AdequateToxicological Data was Found
- 13. Options for Describing Limits of Class 2 Solvents These options are used to describe the limit of Class 2 solvents. Testing should be performed for residual solvents when production or purification processes are known to result in the presence of such solvents. Option 1: By assuming a product mass of 10 g administered daily. Concentration (ppm) = 1000 x PDE / Dose Here, PDE is given in terms of mg/day and dose is given in g/day. No further calculation is necessary provided that the daily dose does not exceed 10 g. Option 2: Products that are administered in doses greater than 10 g per day. Applied by adding the amounts of a residual solvent present in each of the components of the drug product. The sum of the amounts of solvent per day should be less than that given by the PDE.
- 14. Example for Option 2 The permitted daily exposure to acetonitrile is 4.1 mg per day; thus, the Option 1 limit is 410 ppm. The maximum administered daily mass of a drug product is 5.0 g, and the drug product contains two excipients. The composition of the drug product and the calculated maximum content of residual acetonitrile are given in the following table. Excipient 1 meets the Option 1 limit, but the drug substance, excipient 2, and drug product do not meet the Option 1 limit. however, the product meets the Option 2 limit of 4.1 mg per day and thus conforms to the recommendations in this guideline.
- 15. What if the product meets neither the Option 1 nor the Option 2 limit ? The manufacturer could test the drug product to determine if the formulation process reduced the level of acetonitrile. If the level of acetonitrile was not reduced during formulation to the allowed limit, then the manufacturer of the drug product should take other steps to reduce the amount of acetonitrile in the drug product. If all of these steps fail to reduce the level of residual solvent, in exceptional cases the manufacturer could provide a summary of efforts made to reduce the solvent level to meet the guideline value, and provide a risk benefit analysis to support allowing the product to be utilized with residual solvent at a higher level.
- 16. Specifications for class 1 and class 2 residual solvents in active substances A) Class 1 solvents used as starting materials They should be routinely controlled, either in a suitable intermediate or in the final active substance. B) Class 1 solvents present as an impurity It should be NMT 30 % of the specified limit, in a suitable intermediate or in the final active substance. Supporting data should be presented on 6 consecutive pilot scale batches or 3 consecutive industrial scale batches. C) Class 2 solvents used in the last step of the synthesis It should be routinely controlled in the final active substance. D) Class 2 solvents used prior to the last step of the synthesis It should be NMT 10 % of the acceptable concentration limit (e.g., acetonitrile 41 ppm). Supporting data should be presented on 6 consecutive pilot scale batches or 3 consecutive industrial scale batches.
- 17. Analytical Procedures •Residual solvents are typically determined using chromatographic techniques such as gas chromatography. •Any harmonized procedures for determining levels of residual solvents as described in the pharmacopoeias should be used. •Manufacturers would be free to select the most appropriate validated analytical procedure for a particular application. •If onlyClass 3 solvents are present, a nonspecific method such as loss on drying may be used.
- 18. • Gas chromatograph equipped with • Headspace Sampler • Flame Ionization Detector (FID) • Mass-selective Detector (MSD) (optionally)for identification & confirmation
- 19. a G43 Column ACI Limited 19
- 20. HeadspaceVial
- 21. • If we look at the anatomy of a headspace vial we can begin to see the relationship of the vial components and how we can control these parameters to create analytical methods. • Volatile components partition from the sample phase and equilibrate in the vial headspace. ACI Limited
- 22. Principle for analysis of residual solvents (continued…) • Residual solvent analysis by static HS/GC can be enhanced by careful consideration of two basic concepts—partition coefficient (K) and phase ratio (β). • Partition coefficients and phase ratios work together to determine the final concentration of volatile compounds in the headspace of sample vials. • The partition coefficient (K) is defined as the equilibrium distribution of an analyte between the sample and gas phases. Compounds that have low K values will tend to partition more readily into the gas phase, and have relatively high responses and low limits of detection. • The phase ratio (β) is defined as the volume of the headspace over the volume of the sample in the vial. Lower values for β (i.e., larger sample sizes) will yield higher responses for compounds with inherently low K values.
- 23. Principle for analysis of residual solvents (continued…) • Striving for the lowest values for both K and β when preparing samples will result in higher concentrations of volatile analytes in the gas phase and, therefore, better sensitivity ACI Limited
- 24. Overview of Method for Residual SolventTesting • USP <467> is divided into two separate sections based upon sample solubility: water-soluble and water-insoluble articles.The methodology for both types of articles is similar, but the diluent used in both standard and sample preparations differs based upon the solubility of the test article. • The test method consists of three procedures (A, B, and C), that are designed to identify, confirm, and then quantify residual solvents in drug substances and products . • The revised USP <467> method consists of a static headspace extraction coupled with a gas chromatographic separation and flame ionization detection.
- 25. 25
- 26. Procedure A - Identification • Procedure A is the first step in the identification process and is performed on a G43 column to determine if any residual solvents are present in the sample at detectable levels. • First, Class 1 standard and system suitability solutions and Class 2 Mix A standard solutions are assayed under the method-specified operating conditions to establish system suitability. • All peaks in the Class 1 system suitability solution must have a signal-to-noise ratio not less than 3, the Class 1 standard solution must have a 1,1,1- trichloroethane response greater than 5, and the resolution of acetonitrile and dichloromethane must be not less than 1 in the Class 2 Mixture A solution. • When system suitability has been achieved, the test solutions are assayed along with the Class 1 and Class 2 Mixtures A and B standard solutions. If a peak is determined in the sample that matches a retention time and has a greater response than that of a corresponding reference material, then Procedure B is performed for verification of the analyte.
- 27. Identification of residual solvent in sample by comparing with reference standard ACI Limited 27
- 28. Procedure B - Confirmation • Once a residual solvent is identified and found to be above the percent daily exposure limit, Procedure B is performed to confirm analyte identity. • A G16 capillary column is used here as a confirmation column, because it yields an alternate selectivity compared to a G43 column. The same standard and system suitability preparations are used in Procedures A and B. • The system suitability requirements differ here in that the Class 1 standard solution must have a benzene response greater than 5 and the resolution of acetonitrile and cisdichloroethene must not be less than 1 in the Class 2 Mixture A solution, a change from the original version. • If the analyte identified in Procedure A again matches the retention time and exceeds the peak response of the reference materials (with the same exception to 1,1,1-trichloroethane), the analyst must quantify the analyte using Procedure C. 28
- 29. Confirmation of residual solvent in sample by comparing retention time and peak response ACI Limited 29
- 30. Procedure C – Quantification • Once a residual solvent has been identified and verified, Procedure C is used to quantify the analyte by analyzing the sample against compound-specific reference materials. • Individual standards are prepared by diluting the analyte in solution to a concentration of 1/20 of the concentration limit given under concentration limitTable 1 or 2 of the method. • Following the procedure and instrument conditions in either Procedure A or B (whichever provides the most definitive results), a quantifiable result is produced. • For water-insoluble articles, the same procedure is followed, except dimethylformamide or dimethylsulfoxide is used as the diluent. 30
- 31. Confirmation of residual solvent in sample by analyzing the sample against compound-specific reference materials. ACI Limited 31
- 32. Reporting levels of residual solvents •Manufacturers of pharmaceutical products need certain information about the content of residual solvents in excipients or drug substances. •The following statements are given in the ICH Guideline as acceptable examples of the information that could be provided from a supplier of excipients or drug substances to a pharmaceutical manufacturer. OnlyClass 3 solvents are likely to be present. Loss on drying is less than 0.5%. Only Class 2 solvents X,Y, ... are likely to be present. All are below theOption 1 limit. Only Class 2 solvents X,Y, ... and Class 3 solvents are likely to be present. Residual Class 2 solvents are below the Option 1 limit and residual Class 3 solvents are below 0.5%.
- 33. Reporting levels of residual solvents •If Class 1 solvents are likely to be present, they should be identified and quantified. •If solvents of Class 2 or Class 3 are present at greater than their Option 1 limits or 0.5%, respectively, they should be identified and quantified. •Manufacturer could provide a summary of efforts made to reduce the solvent level to meet the guideline value and provide a risk-benefit analysis to support allowing the product to be utilized with residual solvent at a higher level. •Higher levels of residual solvents may be acceptable in certain cases such as short term (30 days or less) or topical application. Justification for these levels should be made on a case by case basis. Example for Residual Solvents Declaration
- 34. Residual Solvents in Pharmaceuticals Exposure limits in this guideline are established by referring to methodologies and toxicity data described in EHC and IRIS* monographs. However, some specific assumptions about residual solvents to be used in the synthesis and formulation of pharmaceutical products should be taken into account in establishing exposure limits: 1) Patients (not the general population) use pharmaceuticals to treat their diseases or for prophylaxis to prevent infection or disease. 2) Residual solvents are unavoidable components in pharmaceutical production and will often be a part of drug products. 3) Residual solvents should not exceed recommended levels except in exceptional circumstances. EHC: Environmental Health Criteria IRIS: Integrated Risk Information System
- 35. 4) Data from toxicological studies that are used to determine acceptable levels for residual solvents should have been generated using appropriate protocols such as those described for example by FDA Red Book and EPA*. FDA Red Book:Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in Food EPA: US Environmental Protection Agency References: Impurities: Guideline for Residual SolventsQ3C(R5) EMA: CVMP/VICH/502/99 Guideline on impurities: residual solvents , Annex I: specifications for class 1 and class 2 residual solvents in active substances
- 36. Glossary Term Meaning Term Meaning ICH INTERNATIONALCONFERENCE ON HARMONISATION LOEL Lowest-Observed Effect Level WHO World Health Organization NOEL No-Observed Effect Level GMP Good Manufacturing Practice PDE Permitted Daily Exposure EHC Environmental Health Criteria TDI Tolerable Daily Intake IRIS Integrated Risk Information System ADI Acceptable Daily Intake IPCS International Program on Chemical Safety USFDA United States Food and Drug Administration USEPA United States Environmental Protection Agency EWG ExpertWorking Group