Advances in Immunotherapy for Non-Small Cell Lung Cancer
- 1. Advances in Immunotherapy for Non-Small Cell Lung Cancer Jarushka Naidoo, MB BCH Assistant Professor of Oncology Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University Bloomberg-Kimmel Institute for Cancer Immunotherapy FLASCO Annual Meeting May 18, 2018
- 2. Disclosures • Research Funding - Merck - AstraZeneca - NIH KL2 • Consulting - AstraZeneca - Bristol Myers Squibb - Takeda • Honoraria - AstraZeneca - Bristol Myers Squibb
- 3. Outline • Brief Introduction to Immunotherapy in NSCLC • First-line Immunotherapy in NSCLC • Immunotherapy for Stage III NSCLC • Future Directions: - Neoadjuvant Studies - Translational Studies
- 4. T T T Lymph node T T T T T PD-L1 GAL9 VISTA LAG3 BTLA4 OX40 GITR HVEM CD137 CD28 Immunotherapy in Cancer The Cancer-Immunity Cycle Adapted from DS Chen et al, Immunity 2012 CTLA4 CTLA4 CTLA4 Chemotherapy Radiation Targeted Therapy Vaccines IFN-alpha GM-CSF Anti-CD40 Anti-CTLA4 Anti-CD137 Anti-OX40 IL2 Anti-CXCL12 Anti-VEGF CAR T-cells Immune Checkpoint mAbs PD-1
- 5. Specificity: virtually infinite antigen recognition Adaptability: based on tumor genetic & epigenetic changes Memory: durable responses even after drug discontinuation Universality: potential anti-tumor effect regardless of tumor type The Human Immune System The Ultimate Anti-cancer Therapy? Tumor Types with Objective Response to Anti-PD-1/PD-L1 Melanoma Non-small cell lung carcinoma Renal Cell carcinoma Urothelial carcinoma Head and Neck carcinoma Merkel Cell carcinoma MSI-high Colorectal carcinoma Biliary Tract carcinoma Ovarian carcinoma Breast carcinoma Anal carcinoma Mesothelioma Gastric adenocarcinoma Hogkins Lymphoma Hepatocellular carcinoma Naidoo et al, Ann Transl Med 2016
- 6. Immunotherapy in NSCLC Second-Line Therapy: Nivolumab vs. Docetaxel 272 patients: 135 (nivolumab); 137 (docetaxel) Brahmer J, et al. N Engl J Med. 2015 Borghaei H, et al. N Engl J Med. 2015 582 patients: 292 (nivolumab); 290 (docetaxel)
- 7. PD-L1 Immunohistochemical assay PD-L1 >50% All patients (PD-L1 >1%) 1034 patients: 345 (pembro 2mg/kg), 346 (pembro 10mg/kg), 343 (docetaxel) Garon EB, et al. N Engl J Med; Herbst RS, et al. Lancet. 2016 Immunotherapy in NSCLC Second-line Therapy: PD-L1+ NSCLC, KEYNOTE studies
- 8. 8 OS= HR, 0.73 (95% CI, 0.60, 0.89) P = 0.0015 Median 11.2 mo (95% CI, 9.3, 12.6) Median 15.6 mo (95% CI, 13.3, 17.6) Atezolizumab Docetaxel Median 7.7 mo (95% CI, 6.3, 8.9) Median 8.9 mo (95% CI, 7.4, 12.8) OS= HR, 0.73 (95% CI, 0.54, 0.98) P = 0.0383 Non-squamous Squamous Months Months Anti-PD-L1 in Second-Line Atezolizumab vs. Docetaxel: OS by histology Rittmeyer A et al. Lancet. 2017;389:255-265
- 9. Gandara DR, et al. bTMB in POPLAR & OAK Atezolizumab Blood TMB subgroups Progression-FreeSurvival(%) • Blood TMB ≥16 population accounted for 27% of biomarker evaluable group (N = 158) • PFS benefit with atezolizumab was observed in the TMB ≥16 subgroup • No prognostic effect was observed: patients with bTMB ≥16 did not have improved PFS compared with patients with bTMB <16 in the docetaxel arm Interaction P = 0.036 Progression-FreeSurvival(%) Blood TMB ≥16 Blood TMB <16 Atezolizumab (N = 216) Docetaxel (N = 209) + Censored Atezolizumab (N = 77) Docetaxel (N = 81) + Censored Months Months Progression-FreeSurvival(%) Gandara DR et al. ESMO 2017
- 10. How to Evaluate for Blood-based TMB Poplar and Oak Studies • A 394 gene-based NGS assay - Retrospective test of plasma samples (phase 2 POPLAR; phase 3 OAK study) • 211/273 samples from POPLAR and 583/797 samples from OAK were evaluable – The association between TMB and efficacy was analyzed and the cut-point of TMB ≥16 was selected based on POPLAR – Validated in OAK bTMB Computational Methodology and Study Design Blood collection, plasma isolation & cfDNA extraction bTMB Sequencing POPLAR (training) OAK (validation) • All base substitutions with ≥0.5% allele frequency • Remove germline polymorphisms & predicted driver mutations Gandara DR et al. ESMO 2017
- 11. Outline • Brief Introduction to Immunotherapy in NSCLC • First-line Immunotherapy in NSCLC • Immunotherapy for Stage III NSCLC • Future Directions: - Neoadjuvant Studies - Translational Studies
- 12. Progression-Free Survival Events, n Median, months HR (95% CI) P Pembro 73 10.3 0.50 (0.37-0.68) <.001 Chemo 116 6.0 62% 50% 0 3 6 9 12 15 18 0 10 20 30 40 50 60 70 80 90 100 Time, months PFS,% No. at risk 154 104 89 44 22 3 1 151 99 70 18 9 1 0 48% 15% ST v1.1 by blinded, independent central review , 2016 Ann Oncol. 2016;27(suppl 6): Abstract LBA8. Reck M, et al. N Engl J Med. 9 Oct 2016. [Epub ahead of print]. Overall Survival 80% 72% 0 3 6 9 12 15 18 21 0 10 20 30 40 50 60 70 80 90 100 Time, monthsOS,%No. at risk 154 136 121 82 39 11 0 151 123 106 64 34 7 0 2 1 70% 54% Events, n Median, months HR (95% CI) P Pembro 44 NR 0.60 (0.41-0.89) .005 Chemo 64 NR DMC recommended stopping the trial because of superior efficacy observed with pembrolizumab Data cut-off: May 9, 2016 Reck M, et al. Ann Oncol. 2016;27(suppl 6): Abstract LBA8. Reck M, et al. N Engl J Med. 9 Oct 2016. [Epub ahead of print Progression-Free Survival 5.7 month PFS benefit HR= 0.5, p<0.001 48% vs. 15% progression-free at 1 year Median OS results not mature HR= 0.6, p=0.005 70% vs. 54% alive at 1 year Reck et al, N Engl J Med 2016 First-line Pembrolizumab in NSCLC KEYNOTE 024: PFS and OS Overall Survival
- 13. Pembro Responders n = 69 Chemo Responders n = 42 TTR, mo median (range) 2.2 (1.4-8.2) 2.2 (1.8-12.2) DOR, mo median (range) NR (1.9+ to 14.5+) 6.3 (2.1+ to 12.6+) First-line Pembrolizumab in NSCLC KEYNOTE 024: ORR Reck et al, N Engl J Med 2016 Objective Response Rate Duration of Response
- 14. Ph III First-Line Pembrolizumab KEYNOTE 042: Study Design Data to be presented ASCO 2018
- 15. First-Line Nivolumab in NSCLC Checkmate 026: OS Socinski et al, ESMO 2016
- 16. TMB as a Biomarker First-Line Nivolumab in NSCLC: PFS High TMB Low/Intermediate TMB Garon et al, ASCO 2017
- 17. First-line PD-1 Monotherapy in NSCLC Summary • Pembrolizumab - Improved survival compared to chemotherapy in advanced NSCLC patients - Relevance: PD-L1 > 50% and in patients PD-L1 > 1% • Nivolumab - Did not improve survival compared to chemotherapy for advanced NSCLC patients whose PD-L1 > 5%
- 18. Pembrolizumab 200 mg Q3W for 2 years + Pemetrexed 500 mg/m2 + Carboplatin AUC 5 mg/mL/min Q3W for 4 cycles Pemetrexed 500 mg/m2 + Carboplatin AUC 5 mg/mL/min Q3W for 4 cycles R (1:1)a N = 123 First-line Pembrolizumab+Chemo in NSCLC KEYNOTE-021 Cohort G: Study Design Pemetrexed 500 mg/m2 Q3W permitted as maintenance therapy Study Population •Untreated stage IIIB or IV nonsquamous NSCLC •No activating EGFR mutation or ALK translocation •Provision of a sample for PD-L1 assessmenta •ECOG PS 0 or 1 •No untreated brain metastases •No ILD or pneumonitis requiring systemic steroids End Points •Primary: ORR (RECIST v1.1 per blinded, independent central review) •Key secondary: PFS: OS, safety, relationship between antitumor activity and PD-L1 TPS Langer et al, Lancet Oncol 2017
- 19. 0 3 6 9 12 15 18 21 24 27 0 10 20 30 40 50 60 70 80 90 100 Time, months OverallSurvival,% 60 57 55 51 46 44 36 22 7 1 63 58 57 51 43 39 29 18 9 0 No. at risk HR= 0.59 77% vs. 69% alive at 1 year Pembro/Chemo vs, Chemo p=0.03 HR= 0.54 Pembro/Chemo vs, Chemo 57% vs. 35% PFS at 1 year p=0.006 Progression-Free Survival Overall Survival First-line Pembrolizumab+Chemo in NSCLC KEYNOTE-021 Cohort G: PFS and OS Langer et al, Lancet Oncol 2017
- 20. Δ24.8% (95% CI, 7.2% 40.9%)‒ P = 0.0029a Pembro + PC Responders n = 34 PC Alone Responders N = 20 Median (range) duration of response, mo NR (1.4+ to 22.7+) NR (2.8 to 23.7+) Ongoing response, % 50 40 First-line Pembrolizumab+Chemo in NSCLC KEYNOTE 021-G: ORR Langer et al, Lancet Oncol 2017
- 21. First-line Pembrolizumab+Chemo in NSCLC KEYNOTE 189: Ph III: Study Design Gandhi et al, NEJM 2018
- 22. Overall SurvivalProgression-Free Survival First-line Pembrolizumab+Chemo in NSCLC KEYNOTE 189: Ph III: PFS and OS HR= 0.49 69% vs. 49% OS at 1 year Pembro/Chemo vs. Chemo p<0.001 HR= 0.52 34% vs. 17% PFS at 1 year Pembro/Chemo vs. Chemo p<0.001 Gandhi et al, NEJM 2018
- 23. First-line Pembrolizumab+Chemo in NSCLC KEYNOTE 189: Ph III: Subgroup Analyses Gandhi et al, NEJM 2018
- 24. First-line Pembrolizumab+Chemo in NSCLC KEYNOTE 189: ORR Gandhi et al, NEJM 2018
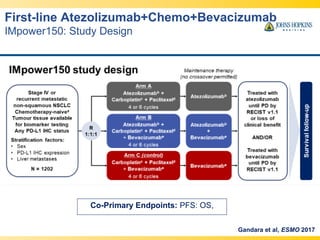
- 25. Co-Primary Endpoints: PFS: OS, First-line Atezolizumab+Chemo+Bevacizumab IMpower150: Study Design Gandara et al, ESMO 2017
- 26. First-line Atezolizumab+Chemo+Bevacizumab IMpower150: PFS and OS Progression-Free Survival Overall Survival Gandara et al, ESMO 2017
- 27. • In advanced non-squamous NSCLC - Carboplatin+Pemetrexed+Pembro is superior to chemotherapy alone, independent of PD-L1 - Carboplatin+Paclitaxel+Atezo+Bevavizumab is superior to chemotherapy + bevacizumab, independent of PD-L1 - Carboplatin+Paclitaxel+Atezo+Bevavizumab benefitted patients with EGFR/ALK alterations • PD-L1 > 50% is relevant to select - Pembrolizumab alone vs. Pembrolizumab + Chemotherapy First-line PD-1+Chemotherapy in NSCLC Summary
- 28. First-line Durvalumab + Tremelimumab in NSCLC MYSTIC study: Design
- 29. First-line Nivolumab+Ipilimumab in NSCLC Phase III Checkmate 227: Study Design Hellmann et al, NEJM 2018
- 30. First-line Nivolumab+Ipilimumab in NSCLC Phase III Checkmate 227: Analytic Plan Hellmann et al, NEJM 2018
- 31. First-line Nivolumab+Ipilimumab in NSCLC PFS in High-TMB NSLC (>10 mut/Mb) Hellmann et al, NEJM 2018
- 32. First-line Nivolumab+Ipilimumab in NSCLC Preliminary OS in High-TMB NSLC (>10 mut/Mb) Hellmann et al, NEJM 2018
- 33. First-line Nivolumab+Ipilimumab in NSCLC ORR and DOR in High-TMB NSLC (>10 mut/Mb) Hellmann et al, NEJM 2018
- 34. • Durvalumab-Tremelimumab - Was no better than chemotherapy in treatment-naïve patients (press release) • Ipilumumab-Nivolumab - Demonstrates superior PFS vs. chemotherapy in patients - with NSCLCs that have a high-TMB (10mut/Mb) First-line PD-1/PD-L1 +CTLA-4 in NSCLC Summary
- 35. Outline • Brief Introduction to Immunotherapy in NSCLC • First-line Immunotherapy in NSCLC • Immunotherapy for Stage III NSCLC • Future Directions: - Neoadjuvant Studies - Translational Studies
- 36. Durvalumab in Stage III NSCLC Phase III PACIFIC Trial: Study Design Antonia et al, NEJM 2017
- 37. Antonia et al, NEJM 2017 Durvalumab in Stage III NSCLC Phase III PACIFIC Trial: PFS
- 38. Durvalumab in Stage III NSCLC Phase III PACIFIC Trial: Safety Antonia et al, NEJM 2017
- 39. • Durvalumab Maintenance Therapy - Is a new standard of care after definitive chemoradiation for stage III NSCLC Stage III NSCLC Summary
- 40. Neoadjuvant Clinical Trials General Principles • Clinical and translational advantage over adjuvant approach • No advances in resectable lung cancers since 2004 • Anti-PD-1/PD-L1 induces deep and durable responses in a subset of patients with advanced NSCLC • Neoadjuvant Anti-PD-1 may: • Induce immunity against micrometastases • Allow for a pre and post PD-1 pathologic assessment • Provide tissue for correlative analyses Forde et al, NEJM 2018
- 41. Slide 3 Neoadjuvant Nivolumab in Resectable NSCLC Study Design Primary Endpoint: Safety (drug-related adverse events 90-days post PD-1/30 days post surgery Feasibility (resection without delay >37 days from pre-planned surgery) Sample Size: 6 patient safety run-in, 20 patients in total Exploratory Endpoints: Pathologic response, RFS, OS, immunologic correlates Forde et al, NEJM 2018
- 42. Neoadjuvant Nivolumab in Resectable NSCLC Patient Characteristics Safety -1 death in the postoperative period, unrelated to study drug Feasibility - All patients underwent surgery without delay - No complications in the postoperative period 22 enrolled 21 treated with pre-op intent 1 withdrew (SCLC histology) 20 resected 1 unresectable Forde et al, NEJM 2018
- 43. Neoadjuvant Nivolumab in Resectable NSCLC Response to Therapy Radiologic Response after 2 doses neoadjuvant nivolumab Pleomorphic NSCLC Pathologic CR Squamous NSCLC Pathologic CR Pathologic Response after 2 doses neoadjuvant nivolumab Major Pathologic Response: <10% viable tumor MPR in 43% (9/21) patients Pre-nivo PD-L1 (>1%) did not correlate with MPR Forde et al, NEJM 2018
- 44. Slide 14 Neoadjuvant Nivolumab in Resectable NSCLC T-cell specific for dominant MANA expand in peripheral blood With thanks K. Smith and D. Pardoll
- 45. Slide 15 With thanks K. Smith and D. Pardoll Neoadjuvant Nivolumab in Resectable NSCLC T-cell specific for dominant MANA expand in peripheral blood
- 46. Slide 16 With thanks K. Smith and D. Pardoll Neoadjuvant Nivolumab in Resectable NSCLC T-cell specific for dominant MANA expand in peripheral blood
- 47. Neoadjuvant Nivolumab in Resectable NSCLC TMB and Neoantigen density associates with MPR With thanks K. Smith and D. Pardoll
- 48. Neoadjuvant Nivolumab in Resectable NSCLC Conclusions • Nivolumab prior to lung cancer resection did not delay time to surgery • There were no unacceptable safety signals with neoadjuvant nivolumab • 43% of tumors had MPRs • Correlative analyses in a subset of tumors demonstrated • Associations between mutation, MANA burden and pathologic response • Identified MANA-specific TCRs in the blood and tumor • Observed temporal increases in MANA-specific TCRs in the peripheral blood post nivolumab: a potential biomarker of response Forde et al, NEJM 2018
- 49. • Neoadjuvant chemoradiation for Resectable Stage IIIA NSCLC - Used for a subset of patients with stage IIIA disease, deemed resectable - Increased rate of pathologic CR, reduced rate of locoregional recurrence1,2 • Immunologic Synergy between PD-1/PD-L1 Blockade and RT - Anti-CTLA-4 diversifies the TCR, which is associated with improved OS3 - Anti-PD-1+RT demonstrates TCR diversification in tumor and systemically4 Neoadjuvant Studies in Resectable NSCLC Future Directions 1. Ettinger et al, JNCCN 2017 2. Albain et al, Lancet 2009 3. Demaria et al, Clin Cancer Res 2005 4. Deng et al, J Clin Investig 2014 4. Twyman-Saint Victor et al, Nature 2015
- 50. Neoadjuvant Immunoradiation in Stage IIIA Resectable NSCLC Cohort 2 opens when safety and feasibility of cohort 1 established IRB: J1772, PI: Naidoo
- 51. JHH Thoracic Oncology Program Julie R. Brahmer, MD Patrick M. Forde, MD David S. Ettinger, MD Ronan J. Kelly, MD MBA Christine L. Hann, MD PhD Benjamin Levy, MD Josephine Feliciano, MD Kristen M. Marrone, MD Jessica Wakefield, MS Rachel Levy, MS Joanne Riemer, RN Cancer Biology Program Valsamo Anagnostou, MD PhD Victor E. Velculescu, MD PhD Department of Radiology Tony Lin, MD Funding Sources Stand Up To Cancer (SU2C) Lungevity IASLC NIH KL2 program JHH Bloomberg Kimmel Institute for Cancer Immunotherapy (BKI) Drew M. Pardoll, MD PhD Elizabeth M. Jaffee, MD PhD Kellie Smith, PhD Suzanne Topalian MD Department of Pathology Tricia Cottrell, MD PhD Janis Taube, MD Peter Illei, MD Colleagues and Collaborators
Editor's Notes
- #5: - Firstly, the interaction between a cancer and the immune system is a complex one, but can be simplified into a stepwise process called the cancer-immunity cycle. - This starts with Tumor ag release through cell death or destruction Uptake and presentation of ag’s by APCs and the generation of an adaptive immune response and ag-specific t-cells in the LN (priming and activation phase) trafficking of ag-specific T-cells to the tme and when they enter TME, recognize cancer cells as foreign via TCR specificity. When the TCR binds to the tumor cells that express known Ag, this induces a specific anti-tumor immune response. This last step is tightly regulated by other inhibitory and activating factors on immune cells and tumor cells, that can potentially be therapeutically targeted. and we can see that theorectically a number of anticancer agents can influence and potentially strengthen different stages of this cancer immunity cycle, in order to generate an immune mediated anti tumor response. At the final stage in this cycle, therapeutic targeting of an inhibitory molecule that has garnered much interest is pd1 and it’s corresponding ligands PDL1 and PD-L2
- #6: - So what makes immunotherapy different to any other kind of therapy we hear about or is under investigation as an anti-cancer agent? There are several unique features of the adaptive immune system in particular that makes it an appealing anti-cancer strategy, Firstly, every T-cell is specific to a particular antigen The immune system is able to evolve and generate new T cell clones in response to tumor evolution and antigen expression Probably the most attractive factor, T-cells have memory- such that is a new antigen or antigen that elicits cross-reactivity with a particular T-cell clone exists, the adaptive immune system will maintain immunity against it, just as vaccines work for infective illnesses And of course we all have an immune system, therefore these agents may not be specific tumor type, and may be able to work across tumor types, if an immune response against the tumor can be elicited And indeed we have seen in clinical trials that clinical trials have provided proof of principle for many of these concepts, with anti-PD-1 therapy
- #7: - As this audience is aware, Dr. Brahmer led the first ph III clin trial of nivolumab compared to standard single agent docetaxel chemotherapy in the second-line squamous NSCLC in CM 017 In this study, patients had a 3m OS benefit, a 1m PFS benefit, and 21% of patients receiving nivo were progression free at 1 year compared to 6% of patients treated with docetaxel. In a subset analysis based on PD-L1 status, it was found that all patients benefitted from nivo, regardless of PD-L1 expression Hossein Borghaei lead a similar study in in the non-sq population in CM 057 study The results of this study were very similar, in that we saw a 3m benefit in OS and 19% of patients were progression free at 1 yr compared to 8% with docetaxel
- #8: Pembro was studied in the large KEYNOTE 010 study in over 1000 pre-treated NSCLC patients These studies included prospective validation of the PD-L1 IHC test using the 22c3 ab In this study PD-L1 positive tumors again demonstrated an OS benefit in favor of pembro compared with chemo in second-line In all patients, the HR for OS was 0.71 for pembro 2, 0.61 for pembro 10 compared with docetaxel Additionally, about 1 third of patients were strongly expressors of pd-l defined as &gt;50% expression on tumor cells In these patients the median OS was 14.9m for pembro 2, 17.3m for pembro 10 and 8.2m for docetaxel
- #9: This study demonstrated a benefit in OS in the first 850 pts, with an approx. 4m benefit in median OS in the non-sq population Similarly, pts with sq NSCLC had a benefit in median OS of approx 1m, and HRs for benefit in OS was similar in both populations, and was ss
- #13: This study met it’s primary endpt, such that pembro demonstrated superior PFS (HR 0.50) over plt-doublet chemotherapy Pembrolizumab also demonstrated superior OS vs. chemotherapy (HR 0.60) In support of these findings the trial was stopped early for superior efficacy demonstrated in favor of pembro
- #14: • Pembrolizumab also provided a substantial improvement in ORR over chemotherapy: with 45% of patients responding compared with 28% with chemo – This included 6 pts who were complete responders with pembrolizumab - Data regarding DOR is still early, reflected by the median DOR NR in the pembro group, compared to 6.3m with docetaxel - It is with this data in mind as well as a manageable safety profile, pembrolizumab has become a new standard of care for advanced NSCLC that expresses &gt;50% levels of PD-L1 in first-line
- #43: This is a pilot IIT run by my colleague Patrick Forde and myself in collaboration with MSKCC, where 20 pts with newly diagnosed resecteble stage IA-IIIA non-bulky NSCLC were given 2 doses of anti-PD-1 prior to surgery. The co-primary endpoints of the study were The study incorporated a 6 patient safety run in And a host of correlative analyses were planned including pre and post Tx large vol blood draws, 6-8 core tissue biopsy samples and assessment of resection specimen and DLNs
- #44: This is a consort diagram of the study which demonstrates that 22 pts enrolled, one withdrew due to SCLC histology, 21 underwent preop tx, 20 proceeded to resection and 1 was unresectable The patient characteristics are summarized in this table and are in keeping with a typical NSCLC population, median age 67, equal no. females and males, mostly ADC histology, and current/former smokers
- #45: Selected patients demonstrates deep responses to therapy with radiologic responses and pathologic complete responses, with evidence of residual viable tumor in the resection specimen after just 2 doses of nivolumab The overall response results for the cohort enrolled are depicted in this waterfall plot, using the pathologic endpoint of MPR: defined as having &lt;10% viable tumor cells in the resection specimen, This has been used as a surrogate endpoint to clinical outcomes for neoadjuvant chemotherapy in nSCLC, and was used as an exploratory response endpoint here In this study, 43%of patients demonstrated an MPR with nivo. We also looked at whether pD-L1 status using the BMS antibody associated with response to rherapy, an using a cut-off of &gt;1% membranous staning as +. No association was seen between + PD-L1 status and MPR
- #46: Looking in closer detail at the MANAfest assays performed in one particular patient enrolled in the neoadjuvant study, 3 T-cell clones were identified that corresponded to a particular MANA within the tumor tissue prior to therapy
- #47: Interestingly, the MANA-specific t-cell clones of interest were only found to comprise 1.6% of the initial tumor sample from primary tumor and LN biopsy prior to neoadjuvant therapy
- #48: - In this study, we observed dynamic changes in the 3 T-cell clones linked to this MANA in response to anti-PD-1, that expanded in the peripheral blood prior to surgery
- #49: Coming back to our neoadjuvant trial of nivo in NSCLC, we saw a positive association between the number of nonsynonymous tumor mutations and MANA density and the development of MPR, regardless of tumor histology And regardless of pd-l1 status
- #50: Therefore in summary
- #51: - From here, we hope to expand on these observations and have expanded the previously mentioned study to evaluate the combination of ipi/nivo in stage IB-IIIA non-bulky patients - In addition, a subset of patients with stage IIIA NSCLC have bulky mediastinal LN and may be treated with trimodality therapy involving chemo,RT and surgery. We also know from preclinical studies that radiation therapy may support anti-tumor immune responses through diversification of the TCR, and in some instances this has correlated with improved clinical outlcomes
- #52: Therefore we will be opening a follow-up study of the combination of an anti-PD-1 antibody durva together with RT and a second cohort exploring the combination of durva/chemo and RT prior to surgery in patients with stage IIIA disease that is amenable to surgical resection. We will aim to perform similar integrated genomic and immunologic analyses in these patients including MANAfest
- #53: - Again thank you to the clinical and translational team at hopkins, and to the conference Organizers, and I would be glad to take questions or hear comments.











![Progression-Free Survival
Events,
n
Median,
months
HR
(95% CI)
P
Pembro 73 10.3 0.50
(0.37-0.68)
<.001
Chemo 116 6.0
62%
50%
0 3 6 9 12 15 18
0
10
20
30
40
50
60
70
80
90
100
Time, months
PFS,%
No. at risk
154 104 89 44 22 3 1
151 99 70 18 9 1 0
48%
15%
ST v1.1 by blinded, independent central review
, 2016
Ann Oncol. 2016;27(suppl 6): Abstract LBA8. Reck M, et al. N Engl J Med. 9 Oct 2016. [Epub ahead of print].
Overall Survival
80%
72%
0 3 6 9 12 15 18 21
0
10
20
30
40
50
60
70
80
90
100
Time, monthsOS,%No. at risk
154 136 121 82 39 11 0
151 123 106 64 34 7 0
2
1
70%
54%
Events,
n
Median,
months
HR
(95% CI)
P
Pembro 44 NR 0.60
(0.41-0.89)
.005
Chemo 64 NR
DMC recommended stopping the trial because of
superior efficacy observed with pembrolizumab
Data cut-off: May 9, 2016
Reck M, et al. Ann Oncol. 2016;27(suppl 6): Abstract LBA8. Reck M, et al. N Engl J Med. 9 Oct 2016. [Epub ahead of print
Progression-Free Survival
5.7 month PFS benefit
HR= 0.5, p<0.001
48% vs. 15% progression-free at 1 year
Median OS results not mature
HR= 0.6, p=0.005
70% vs. 54% alive at 1 year
Reck et al, N Engl J Med 2016
First-line Pembrolizumab in NSCLC
KEYNOTE 024: PFS and OS
Overall Survival](https://image.slidesharecdn.com/naidoo-180508151838/85/Advances-in-Immunotherapy-for-Non-Small-Cell-Lung-Cancer-12-320.jpg)