alcohols, phenols and ethers (2).pptx ...
- 1. Alcohols, phenols and ethers Alcohols and phenols are formed when a hydrogen atom in a hydrocarbon, aliphatic or aromatic respectively is replaced by –OH group. In ethers The H atom in a hydrocarbon is replaced by alkoxy/ aryloxy group. (-O-R/ Ar-O) group
- 2. Classification of Alcohols • Monohydric alcohols are classified according to the hybridization of the Carbon atom to which hydroxyl group is attached. 1. Compounds containing C(sp3 )- OH bond. They are further classified as 1. primary, secondary and tertiary alcohols- depending on whether –OH group is attached to 10 , 20 or 30 Carbon 2. Allylic alcohols : – OH group is attached to sp3 hybridized carbon next to carbon-carbon double bond. Eg: CH2= CH –CH2OH 3. Benzylic alcohols: : – OH group is attached to sp3 hybridized carbon next to an aromatic ring.
- 3. • Compounds containing C(sp2 ) –OH bond: They are classified as 1. Vinylic alcohols: OH group bonded to carbon-Carbon double bond. Eg: CH2 = CH-OH 2. Phenols
- 4. Nomenclature of alcohols • The common name of alcohol is derived from the common name of alkyl group and adding the word alcohol to it • Eg: CH3-OH is methyl alcohol • According to IUPAC system, The name of the alcohol is derived from the name of alkane from which the alcohol is derived by substituting ‘e’ of alkane with suffix ‘ol’ The positions of substituents are indicated by numerals. Longest C chain is selected and numbered from the end near to OH group.
- 6. • Cyclic alcohols are named using the prefix cyclo and considering the – OH group attached to C-1 • Cyclohexanol • 2-methylcyclopentanol
- 7. • The simplest hydroxyl derivative of benzene is phenol. It is its common name and accepted IUPAC name. As structure of phenol involves a benzene ring, in its substituted compounds the terms ortho (1,2 disubstituted), meta (1,3- disubstituted) and para(1,4- disubstituted) are often used in the common names.
- 8. Common names and IUPAC names of some ethers • Common names of ethers are derived from the names of alkyl/aryl groups written in separate words in alphabetical order and adding the word ether at the end. For example: CH3-O- C2H5 is ethyl methyl ether. If both the alkyl groups are the same, the prefix ‘di’ is added before the alkyl group. C2H5OC2H5 is diethyl ether. According to IUPAC system ethers are regarded as hydrocarbon derivatives in which a hydrogen atom is replaced by an –OR or –OAr group where R and Ar represent alkyl and aryl groups respectively. The larger group is chosen as the parent hydrocarbon. They are named as alkoxy alkane.
- 10. Structure of functional groups • In alcohols the oxygen of the OH group is attached to carbon by sigma bond formed by the overlap of a sp3 hybridized orbital of carbon with a sp3 hybridized orbital of oxygen. • The C-O-H bond angle in alcohols is slightly less than the tetrahedral angle.(1090 28’). It is due to the repulsion between the unshared electron pairs of oxygen. • In phenols –OH group is attached to sp2 hybridized carbon of an aromatic ring. The Carbon Oxygen bond length in phenol is slightly less than that in methanol. This is due to the partial double bond character on account of resonence. And sp2 hybridized Carbon to which H is attached. • In ethers the four electron pairs. Two bonded and two lone pairs of electrons on oxygen are arranged approximately in a tetrahedral arrangement. Bond angle is slightly greater than the tetrahedral angle due to the repulsive interaction between two bulky (-R) groups. The C-O bond length is almost the same as in alcohols.
- 11. Preparation of alcohols – From alkenes 1. By acid catalyzed hydration: Alkenes react with water in the presence of acid as catalyst to form alcohols. In case of unsymmetrical alkenes, the addition reaction takes place in accordance with Markovnikov’s rule. CH3-CH=CH2 + H2O H+ CH3-CH(OH)- CH3 2. By hydroboration- oxidation:Diborane(BH3)2 reacts with alkenes to give trialkyl boranes as addition product. This is oxidized to alcohol by hydrogen peroxide in the presence of aq.NaOH
- 12. Preparation of alcohols- from carbonyl compounds 1. Aldehydes and ketones are reduced to the corresponding alcohols by addition of hydrogen in the presence of catalysts. (catalytic hydrogenation) Catalysts used are Ni, Pt, Pd. Alcohols are also prepared by treating aldehydes and ketones with NaBH4 or LiAlH4. Aldehydes yield primary alcohol whereas ketones give secondary alcohol. 2. Carboxylic acids are reduced to primary alcohols in excellent yield by LiAlH4, a strong reducing agent. (expensive) commercially acids are converted to esters. Esters on catalytic hydrogenation gives alcohols. (H2/Pt)
- 13. • R CHO + H2 Pd R-CH2OH R-CO-R’ NaBH4 R-CH(OH)-R’ • R-COOH LiAlH4/ H2O R-CH2-OH • R-COOH R’-OH/H+ RCOOR’ H 2/Pt R-CH2OH + R’OH
- 14. From Grignard reagents • Alcohols are produced by the reaction of Grignard reagents with aldehydes and ketones. The first step is the nucleophilic addition of Grignard reagent to the carbonyl group to form an adduct. Hydrolysis of adduct yields alcohol. The reaction of Grignard reagents with methanal (formaldehyde) produces primary alcohol Grignard reagents with other aldehydes produce secondary alcohols Grignard reagents react with ketones to form tertiary alcohols
- 16. Preparation of Phenols 1. From haloarenes: Chlorobenzene is fused with NaOH at 623 K and 320 atmospheric pressure. Sodium phenoxide is formed. Acidification of sodium phenoxide gives phenol.
- 17. Preparation of Phenols From benzene sulphonic acid • Benzene is sulphonated with oleum and benzene sulphonic acid so formed is converted to sodium phenoxide by heating with molten sodium hydroxide. Acidification of the sodium salt gives phenol
- 18. Preparation of Phenols-From diazonium salts • A diazonium salt is formed by treating an aromatic primary amine with nitrous acid.(NaNO2 + HCl) at 273 – 278 K. Diazonium salts are hydrolysed to phenols by warming with water.
- 19. Preparation of Phenols-From cumene • Cumene is isopropyl benzene. It is oxidized in the presence of air to cumene hydroperoxide. It is converted to phenol and acetone by treating it with dilute acid.
- 20. Physical properties • The boiling points of alcohols and phenols increase with increase in the number of Carbon atoms( increase in Van der Waal’s forces.) In alcohols the boiling points decrease with increase in branching in the carbon chain. ( Because of decrease in Van der Waal’s forces with decrease in surface area. • The boiling points of alcohols and phenols are higher in comparison to other classes of compounds- namely hydrocarbons, ethers, haloalkanes and haloarenes of comparable molecular masses. The high boiling points of alcohols are mainly due to the presence of intermolecular hydrogen bonding. • Solubility of alcohols and phenols in water is due to their ability to form hydrogen bonds with water molecules. The solubility decreases with increase in the size of alkyl/aryl groups.
- 21. Hydrogen bonding in alcohols and phenols
- 22. Chemical reactions • Alcohols are versatile compounds. They react both as nucleophiles and electrophiles. • Alcohols as nucleophiles: The bond between O-H is broken when alcohols react as nucleophiles. • Protonated alcohols act as electrophiles: The bond between C-O is broken when they react as electrophiles.
- 23. a. Reaction involving the cleavage of O-H bond 1. Acidity of alcohols and phenols: • Alcohols and phenols react with active metals such as sodium, potassium, and aluminium to yield corresponding alkoxides/ phenoxides and hydrogen. • Phenols react with aqueous NaOH to form sodium phenoxide. TheseThese reactions show the acidic nature of alcohols and phenols
- 24. Acidity of alcohols: • The acidic nature of alcohols is due to the polar nature of O-H bond. An electron releasing group (-CH3, -C2H5) increases electron density on oxygen tending to decrease the polarity of O-H bond. This decreases the acid strength. • Alcohols are weaker acids than water. The following reaction shows water is a better proton donor( better acid)
- 25. Acidity of phenols • The reaction of phenol with aqueous sodium hydroxide indicates that phenols are stronger acids than alcohols and water. • The OH group of phenol is attached to sp2 hybridized Carbon of benzene ring which is electron withdrawing. This increases the polarity of –OH bond and results in the increase in ionization of phenols than alcohols. Also phenoxide ion is resonence stabilized. • In substituted phenols, the presence of electron withdrawing groups (-NO2) enhances the acid strength of phenol. These groups stabilizes the phenoxide ion. The effect is more pronounced when such a group is present at ortho para position. Eg: Orthonitrophenol is stronger than phenol. • Electron releasing groups (such as alkyl groups) decreases the stability of phenoxide ion resulting in the decrease of acid strength. Eg: cresols are less acidic than phenol
- 26. 2. Esterification • Alcohols and phenols react with carboxylic acids, acid chlorides, and acid anhydrides to form esters. • Eg: acetylation of salicylic acid produces aspirin. Aspirin possesses analgesic, anti inflamatory, and antipyretic properties.
- 27. Reaction involving cleavage of C-O bond • The reactions involving cleavage of C-O bond take place only in alcohols. Phenols show this type of reaction only with zinc. 1. Reaction with hydrogen halides: Alcohols react with hydrogen halides to form alkyl halides. The reactivity orders are HI > HBr > HCl > HF ( for a given alcohol) tertiary> secondary > primary ( for a given halogen acid ) R-OH + HX R-X + H2O the difference in reactivity of three classes of alcohols with HCl distinguishes them from one another.(Leucas test)
- 28. Leucas test to distinguish between primary, secondary and tertiary alcohols • Leucas reagent is conc. HCl + ZnCl2. • Tertiary alcohols react with Leucas reagent to give immediate turbidity. Secondary alcohols produce turbidity after about five minutes and primary alcohols do not produce turbidity at room temperature. • Turbidity indicates the formation of alkyl halides as the product as they are insoluble compounds.
- 29. Reaction with phosphorus halides • Alcohols are converted to alkyl halides by the reaction with phosphorus halides. 3 R-OH + PX3→ 3 R-X + H3PO3
- 30. Dehydration • Alcohols undergo dehydration ( removal of a molecule of water) to form alkenes on treating with protic acic. Eg: Conc. H2SO4 or H3PO4. • Example: Ethanol undergoes dehydration by heating it with conc. H2SO4 at 443 K to give ethene.
- 31. Mechanism of dehydration of alcohols • Step 1: formation of protonated alcohol. • Step 2: Formation of carbocation. • Step:3: formation of ethene by the elimination of proton.
- 32. Oxidation • During oxidation of alcohols, a carbon- oxygen double bond is formed with the cleavage of O-H and C-H bonds. ( these reactions are also called dehydrogenation reaction as it involves loss of dihydrogen from an alcohol molecule. • Depending on the oxidizing agent used a primary alcohol is oxidized into an aldehyde which is then oxidized to a carboxylic acid. R-CH2OH [O] R-CHO [O] R COOH • Strong oxidizing agents such as acidified potassium permanganate are used to get carboxylic acids from alcohols directly. • CrO3 in anhydrous medium is used as an oxidizing agent for the isolation od aldehydes. A better reagent for the oxidation of primary alcohols to aldehydes in good yield is pyridinium chlorochromate (PCC). • Secondary alcohols are oxidized to ketones by chromic anhydride. (CrO3) • Tertiary alcohols do not undergo oxidation reaction. Under drastic conditions they undergo oxidation to give a mixture of carboxylic acids with lesser number of carbon atoms.
- 33. Catalytic dehydrogenation of alcohols • When vapours of primary or secondary alcohol are passed over heated copper at 573 K dehydrogenation takes place and an aldehyde or ketone is formed. • Tertiary alcohols when passed over heated copper catalyst at 573 K undergo dehydration.
- 34. Reactions of phenols 1. Electrophillic substitution reactions. • The –OH group attached to benzene ring activates the benzene ring towards electrophilic substitution reactions. Also it directs the incoming group to ortho and para positions in the ring as these position become electron rich due to the resonance effect caused by –OH group.
- 35. Electrophillic substitution reactions of phenol 1. Nitration: With dilute nitric acid at low temperature (298 K) phenol gives a mixture of ortho and para nitro phenols. Orto and para nitro phenols can be separated by steam distillation. O- nitrophenol is steam volatile due to intramolecular hydrogen bonding .while p-nitrophenol is less volatile due to intermolecular hydrogen bonding which causes association of molecules.
- 36. • Nitration of phenol with conc. Nitric acid With conc. Nitric acid, phenol is converted into 2,4,6- trinitrophenol.(picric acid)
- 37. 2. Halogenation • On treating phenol with bromine, different reaction products are formed under different experimental conditions. • When the reaction is carried out in solvents of low polarity such as CHCl3, or CS2 at low temperature, monobromophenols are formed. • When phenol is treated with bromine water 2, 4, 6 tribromophenol is formed as a white ppt.
- 39. Kolbe’s reaction • Phenol is treated with sodium hydroxide to give sodium phenoxide. Sodium phenoxide when treated with CO2 followed by acidification gives salicylic acid (2-hydroxybenzoic acid) Note: phenoxide ion is more reactive than phenol. The reaction is electrophilic substitution reaction as CO2 is a weak electrolyte.
- 40. Riemer Tiemann reaction • On treating phenol with chloroform in the presence of sodium hydroxide, a –CHO group is introduced at the ortho position of benzene ring. This reaction is known as Riemer –Tiemann reaction. The final product formed is salicylaldehyde. •
- 41. Reaction of phenol with zinc dust • Phenol is converted to benzene on treating with zinc dust.
- 42. Oxidation • Oxidation of phenol with chromic acid (Na2Cr2O7 and H2SO4 )produces a conjugated diketone known as benzoquinone.
- 43. Commercially important alcohols 1. Methanol: Methanol, CH3OH also known as wood spirit was produced by distructive distillation of wood. Now most of the methanol is produced by catalytic hydrogenation of carbon monoxide at high pressure and temperature and in the presence of ZnO-Cr2O3 catalyst. Methanol is a colourless liquid, highly poisonous in nature. Ingestion of even small quantities of methanol can cause blindness and large quantities causes even death. Methanol is used as a solvent in paints, varnishes and chiefly for making formaldehyde. It is added to ethanol to make denatured alcohol.
- 44. 2. Ethanol: Ethanol is obtained by fermentation of molasses or fruits like grapes. Yeast acts on sugar in molasses and convert it to glucose and fructose by the enzyme invertase. Glucose and fructose undergo fermentation in the presence of another enzyme, zymase (from yeast) to give ethyl alcohol. Fermentation takes place in anaerobic conditions( in the absence of air). Carbon dioxide is released during fermentation. The action of zymase is inhibited once the percentage of alcohol formed exceeds 14%. Ethanol is a colourless liquid. It is used as a solvent in paint industry and in the preparation of a number of carbon compounds. The commercial alcohol is made unfit for drinking by mixing it with some copper sulphate ( to give it a colour) and pyridine( a foul smelling liquid). It is also known as denatured alcohol.
- 45. Ethers • Preparation: 1. From ethanol by dehydration Ethanol is dehydrated in the presence of sulphuric acid at 413 K to give diethyl ether ( ethoxy ethane).
- 46. 2. Williamson’s Ether synthesis Alkyl halide reacts with sodium alkoxide to give ether. • Ethers containing substituted alkyl groups ( secondary or tertiary can also be prepared by this method. The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide. • Phenols are also converted to ethers by this method. In this, phenol is used as the phenoxide moiety.
- 47. Physical properties • The C-O bond in ethers are polar and thus, have a net dipole moment. The weak polarity of ethers do not appreciably affect their boiling points which are comparable with those of alkanes of comparable molecular masses., but are much lower than alcohols. • The large difference in the boiling points of alcohols and ethers is due to the presence of hydrogen bonding in alcohols.
- 48. Chemical Reactions 1. Cleavage of C-O bond in ethers: Ethers are the least reactive of the functional groups. The cleavage of C-O bond in ethers takes place under drastic conditions with excess of hydrogen halides. R-O-R + HX → RX + R-OH R-OH + HX → RX + H2O • Alkyl aryl ethers are cleaved at the alkyl- oxygen bond due to the more stable aryl- oxygen bond.
- 49. Electrophillic substitution reactions • The alkoxy group (-OR) is ortho, para directing and activates the aromatic ring towards electrophilic substitution in the same way as phenol. 1. Halogenation: Phenyl alkyl ethers undergo usual halogenation in the benzene ring. Eg: anisole undergoes bromination with bromine in ethanoic acid even in the absence of iron (III) bromide catalyst. (FeBr3). This is due to the activation of benzene ring by the methoxy group. Para isomer is obtained in 90% yield.
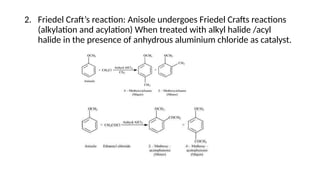
- 50. 2. Friedel Craft’s reaction: Anisole undergoes Friedel Crafts reactions (alkylation and acylation) When treated with alkyl halide /acyl halide in the presence of anhydrous aluminium chloride as catalyst.
- 51. Uses of ethers • A solvent for carrying out an organic reaction.( wurtz reaction, Grignard reagent etc. • As an industrial solvent. • An anesthetic • As a refrigerent
- 52. Questions from previous Q.P. (one 2 mark and one 5 mark) 1. Explain Kolbe’s reaction with equation. 2. Explain the mechanism of acid catalyzed dehydration of ethanol to ethene. 3. How do you prepare methoxy ethane by Williamson’s ether synthesis? 4. How does phenol react with conc nitric acid? 5. Explain Williamson’s reaction? Write the general equation for the preparation of ether by Williamson’s ether synthesis. 6. Among alcohols and phenols which one is more acidic. Give reason.
- 53. 6. What is the action of bromine in ethanoic acid on anisole? Give equation? 7. What is the effect of the following groups on the acidity of phenol?-CH3 , - NO2. 8. Name the product formed when phenol reacts with acidified solution of Na2Cr2O7? Give equation. 9. How is phenol prepared from aniline? Give the equation. 10. How does anisole react with methyl chloride? 11. Complete the following reactions: 1). R-CH2OH Cu/573 K 2). CH3- CH=CH2 H+ 12. How do you prepare methoxy ethane by Williamson’s ether synthesis?
- 54. 13. How is phenol manufactured by cumene process? 14. How does phenol react with zinc dust. Write the equation.































![Oxidation
• During oxidation of alcohols, a carbon- oxygen double bond is formed with the cleavage
of O-H and C-H bonds. ( these reactions are also called dehydrogenation reaction as it
involves loss of dihydrogen from an alcohol molecule.
• Depending on the oxidizing agent used a primary alcohol is oxidized into an aldehyde
which is then oxidized to a carboxylic acid. R-CH2OH [O]
R-CHO [O]
R
COOH
• Strong oxidizing agents such as acidified potassium permanganate are used to get
carboxylic acids from alcohols directly.
• CrO3 in anhydrous medium is used as an oxidizing agent for the isolation od aldehydes. A
better reagent for the oxidation of primary alcohols to aldehydes in good yield is
pyridinium chlorochromate (PCC).
• Secondary alcohols are oxidized to ketones by chromic anhydride. (CrO3)
• Tertiary alcohols do not undergo oxidation reaction. Under drastic conditions they
undergo oxidation to give a mixture of carboxylic acids with lesser number of carbon
atoms.](https://image.slidesharecdn.com/alcoholsphenolsandethers2-250504060651-113268d0/85/alcohols-phenols-and-ethers-2-pptx-32-320.jpg)