Computational Enzymology of Ribozymes (from metal-ion to nucleobase catalysis and back ?)
- 1. Computational Enzymology of Ribozymes from metal-ion to nucleobase catalysis and back ? Laboratoire ARN, RNP, structure-fonction-maturation, Enzymologie Moléculaire et Structurale ( AREMS ) Fabrice Leclerc
- 2. Downloaded from cshperspectives.cshlp.org on January 9, 2012 - Published by Cold Spring Harbor Laboratory Press Schrum et al., 2010; Meierhenrich et al., 2012 J.P. Schrum, T.F. Zhu, and J.W. Szostak Protocells & RNA Figure 1. A simple protocell model based on a replicating vesicle for compartmentalization, and a replicating • containing oligoribonucleotides genome to encode heritable information. A complex environment provides lipids, nucleotides capable of biological-like behaviors (MM. equilibrating across the membrane bilayer, and sources of energy (left), which leads to subsequent replication of the genetic material and growth of the protocell (middle), and finally protocellular division through Hanczyc: Phil. Trans. R. Soc. B. physical and chemical processes (right). (Reproduced from Mansy et al. 2008 and reprinted with permission from Nature Publishing #2008.) us to reconstruct plausible pathways and scenar- tial function of creating an internal environment ios for the origin of life. within which genetic materials can reside and • from “protocells” with The term protocell has been used loosely metabolic activities can take place without being to refer to primitive cells or to the first cells. lost to the environment. Modern cell mem- Here we will use the term protocell to refer spe- branes are composed of complex mixtures of cifically to cell-like structures that are spatially amphiphilic molecules such as phospholipids, delimited by a growing membrane boundary, sterols, and many other lipids as well as diverse • to “modern” cells and that contain replicating genetic informa- proteins that perform transport and enzymatic tion. A protocell differs from a true cell in that functions. Phospholipid membranes are stable the evolution of genomically encoded advanta- under a wide range of temperature, pH, and (JW. Szostak) geous functions has not yet occurred. With a salt concentration conditions. Such membranes genetic material such as RNA (or perhaps one are extremely good permeability barriers, so that of many other heteropolymers that could pro- modern cells have complete control over the vide both heredity and function) and an appro- uptake of nutrients and the export of wastes priate environment, the continued replication through the specialized channel, pump and 2011, ...) of a population of protocells will lead inevitably pore proteins embedded in their membranes. to the spontaneous emergence of new coded A great deal of complex biochemical machinery functions by the classical mechanism of evolu- is also required to mediate the growth and divi- tion through variation and natural selection. sion of the cell membrane during the cell cycle. Once such genomically encoded and therefore The question of how a structurally simple proto- heritable functions have evolved, we would cell could accomplish these essential membrane • ... consider the system to be a complete, living bio- functions is a critical aspect of understanding logical cell, albeit one much simpler than any the origin of cellular life. modern cell (Szostak et al. 2001). Vesicles formed by fatty acids have long been studied as models of protocell membranes BACKGROUND (Gebicki and Hicks 1973; Hargreaves and Deamer 1978; Walde et al. 1994a). Fatty acids Membranes as compartment boundaries
- 3. RNA Chemicals • chemical components of RNA: nucleobases (Powner et al., 2010), sugars (Cocinero et al., Reviews U. J. Meierhenrich et al. 2011) DOI: 10.1002/anie.200905465 The Origin of Life On the Origin of Primitive Cells: From Nutrient Intake to Elongation of Encapsulated Nucleotides Uwe J. Meierhenrich,* Jean-Jacques Filippi, Cornelia Meinert, Pierre Vierling, and Jason P. Dworkin • from “primitive” (TNA: H. Yu Keywords: Dedicated to Professor Wolfram H.-P. amphiphiles · liposomes · micelles · Thiemann nucleotides · vesicles et al., 2012) to “modern” sugar- phosphate backbones • ... • to RNA Angewandte Chemie
- 4. RNA: Information,Catalysis • from self-replicators (JW Szostak, 2012) to self-catalysts and “modern” ribozymes • from RNA to protein catalysts • from the RNA to the ‘protein’ world (activation of GTPases: Bange et al., 2011) • from RNA to DNA genetic information (non-coding RNAs in “modern” genomes)
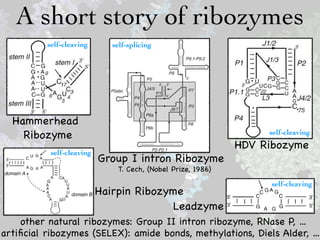
- 5. A short story of ribozymes self-cleaving self-splicing Hammerhead Ribozyme self-cleaving HDV Ribozyme self-cleaving Group I intron Ribozyme T. Cech, (Nobel Prize, 1986) self-cleaving Hairpin Ribozyme Leadzyme other natural ribozymes: Group II intron ribozyme, RNase P, ... artificial ribozymes (SELEX): amide bonds, methylations, Diels Alder, ...
- 6. Self-Splicing / Self-Cleaving 5' 5' Ni Ni ribozyme O O ribozyme O O 4' 4' 3' O O 2' H 3' O O 2' H (OH-) (H2O) (OH-) (H2O) Mg2+ P P Mg2+ 5' O OR Mg2+ Mg2+ O OR Ni+1 OS Ni+1 RO(-)H 5' OS O2' O 3' O2' O 3' Mg2+ H O R4' H O R4' SN2(P) reaction 5' O Ni (“in-line”) 5' O4' Ni His12 O 3' O O 2' H Asp424 O4' 5' P OR B(-) N NH 3' O O 2' H (OH-) Ni+1 O (H2O) Mg2+ OS P O 2' O 3' AH(+) Mg2+ O OR Ni+1 OS RO(-)H H O R4' 5' O2' O 3' Mg2+ Glu357 H O R4' His119 N H RNase A 3’-5’-exonuclease HN
- 7. A prototype for RNA Catalysis “Minimum” Hammerhead Ribozyme substrate/enzyme cleavage site Canonical 2D Structure folded 2D Structure Scott, 1999 Scott et al., Science, 1996
- 8. One-Metal Ion Models 5' Ni NMR model (inactive) O O4' 3' O O 2' H 5' P B(-) O OR Ni+1 OS O 2' O 3' AH(+) H O R4' 630 Nucleic Acids Research, 2005, Vol. 33, No. 2 Mg2+ Ca2+, Mn2+, Co2+, Cd2+ more readily among CN of 4, 5 or 6. It should be noted cobalt hexamine fails to induce activity with either class II or class IV ribozymes (data not shown), which is consistent with the inner sphere coordination of the metal ion. The characteristics described previously are also related to the ‘hard’ or ‘soft’ character of metals. In describing metals, hard generally indicates an electron cloud that is difficult to deform and has low polarization potential, while soft generally indicates an easily deformable electron cloud that permits high polarization and favors bonding interactions of a more covalent character. Figure 8D plots Z/r versus I2, the second ionization constant, and is one way of representing hard versus soft nature (32). It is striking from this figure that the Class II switch has a binding pocket that recognizes a cluster of ‘borderline’ metals (and Cd2+ which is considered a soft metal). This characteristic is perhaps best for explaining the high level of discrimination against Mg2+, while allowing a cluster of borderline metals to act as activators. It may be that relatively small binding motifs can be created, which recogn- ize metals based on their hard-soft character. experimental: Dahm & Uhlenbeck, 1993 The commonality of divalent metal binding pockets Zivarts et al., NAR, 2005 in RNAtheoretical: Torres et al., 2003 The particular type of metal-binding pockets that presumably
- 9. One-Metal Ion Models 5' Ni NMR model (inactive) O O4' 3' O O 2' H 5' P B(-) -HO O OR Ni+1 Mg2+ OS O 2' O 3' AH(+) H O R4' 630 Nucleic Acids Research, 2005, Vol. 33, No. 2 Mg2+ Ca2+, Mn2+, Co2+, Cd2+ more readily among CN of 4, 5 or 6. It should be noted cobalt hexamine fails to induce activity with either class II or class IV ribozymes (data not shown), which is consistent with the inner sphere coordination of the metal ion. The characteristics described previously are also related to the ‘hard’ or ‘soft’ character of metals. In describing metals, hard generally indicates an electron cloud that is difficult to deform and has low polarization potential, while soft generally indicates an easily deformable electron cloud that permits high polarization and favors bonding interactions of a more covalent character. Figure 8D plots Z/r versus I2, the second ionization constant, and is one way of representing hard versus soft nature (32). It is striking from this figure that the Class II switch has a binding pocket that recognizes a cluster of ‘borderline’ metals (and Cd2+ which is considered a soft metal). This characteristic is perhaps best for explaining the high level of discrimination against Mg2+, while allowing a cluster of borderline metals to act as activators. It may be that relatively small binding motifs can be created, which recogn- ize metals based on their hard-soft character. experimental: Dahm & Uhlenbeck, 1993 The commonality of divalent metal binding pockets Zivarts et al., NAR, 2005 in RNAtheoretical: Torres et al., 2003 The particular type of metal-binding pockets that presumably
- 10. One-Metal Ion Models 5' Ni NMR model (inactive) O O4' 3' O O 2' H 5' P B(-) O OR Ni+1 OS O 2' O 3' AH(+) H O R4' 630 Nucleic Acids Research, 2005, Vol. 33, No. 2 Mg2+ Ca2+, Mn2+, Co2+, Cd2+ more readily among CN of 4, 5 or 6. It should be noted cobalt hexamine fails to induce activity with either class II or class IV ribozymes (data not shown), which is consistent with the inner sphere coordination of the metal ion. The characteristics described previously are also related to the ‘hard’ or ‘soft’ character of metals. In describing metals, hard generally indicates an electron cloud that is difficult to deform and has low polarization potential, while soft generally indicates an easily deformable electron cloud that permits high polarization and favors bonding interactions of a more covalent character. Figure 8D plots Z/r versus I2, the second ionization constant, and is one way of representing hard versus soft nature (32). It is striking from this figure that the Class II switch has a binding pocket that recognizes a cluster of ‘borderline’ metals (and Cd2+ which is considered a soft metal). This characteristic is perhaps best for explaining the high level of discrimination against Mg2+, while allowing a cluster of borderline metals to act as activators. It may be that relatively small binding motifs can be created, which recogn- ize metals based on their hard-soft character. experimental: Dahm & Uhlenbeck, 1993 The commonality of divalent metal binding pockets Zivarts et al., NAR, 2005 in RNAtheoretical: Torres et al., 2003 The particular type of metal-binding pockets that presumably
- 11. One-Metal Ion Models 5' Ni NMR model (inactive) O O4' 3' O O 2' H 5' P B(-) OH2 O OR Ni+1 Mg2+ OS O 2' O 3' AH(+) H O R4' 630 Nucleic Acids Research, 2005, Vol. 33, No. 2 Mg2+ Ca2+, Mn2+, Co2+, Cd2+ more readily among CN of 4, 5 or 6. It should be noted cobalt hexamine fails to induce activity with either class II or class IV ribozymes (data not shown), which is consistent with the inner sphere coordination of the metal ion. The characteristics described previously are also related to the ‘hard’ or ‘soft’ character of metals. In describing metals, hard generally indicates an electron cloud that is difficult to deform and has low polarization potential, while soft generally indicates an easily deformable electron cloud that permits high polarization and favors bonding interactions of a more covalent character. Figure 8D plots Z/r versus I2, the second ionization constant, and is one way of representing hard versus soft nature (32). It is striking from this figure that the Class II switch has a binding pocket that recognizes a cluster of ‘borderline’ metals (and Cd2+ which is considered a soft metal). This characteristic is perhaps best for explaining the high level of discrimination against Mg2+, while allowing a cluster of borderline metals to act as activators. It may be that relatively small binding motifs can be created, which recogn- ize metals based on their hard-soft character. experimental: Dahm & Uhlenbeck, 1993 The commonality of divalent metal binding pockets Zivarts et al., NAR, 2005 in RNAtheoretical: Torres et al., 2003 The particular type of metal-binding pockets that presumably
- 12. One-Metal Ion Models 5' Ni NMR model (inactive) O O4' 3' O O 2' H 5' P B(-) O OR Ni+1 OS O 2' O 3' AH(+) H O R4' OH2 630 Nucleic Acids Research, 2005, Vol. 33, No. 2 Mg2+ Mg2+ Ca2+, Mn2+, Co2+, Cd2+ more readily among CN of 4, 5 or 6. It should be noted cobalt hexamine fails to induce activity with either class II or class IV ribozymes (data not shown), which is consistent with the inner sphere coordination of the metal ion. The characteristics described previously are also related to the ‘hard’ or ‘soft’ character of metals. In describing metals, hard generally indicates an electron cloud that is difficult to deform and has low polarization potential, while soft generally indicates an easily deformable electron cloud that permits high polarization and favors bonding interactions of a more covalent character. Figure 8D plots Z/r versus I2, the second ionization constant, and is one way of representing hard versus soft nature (32). It is striking from this figure that the Class II switch has a binding pocket that recognizes a cluster of ‘borderline’ metals (and Cd2+ which is considered a soft metal). This characteristic is perhaps best for explaining the high level of discrimination against Mg2+, while allowing a cluster of borderline metals to act as activators. It may be that relatively small binding motifs can be created, which recogn- ize metals based on their hard-soft character. experimental: Dahm & Uhlenbeck, 1993 The commonality of divalent metal binding pockets Zivarts et al., NAR, 2005 in RNAtheoretical: Torres et al., 2003 The particular type of metal-binding pockets that presumably
- 13. ound water in the fully hydrated La3ϩ ion, the low kobs for Two-Metal Ion Models cleavage reaction involving the La3ϩ ion in both positions not compatible with the observed correlation between the a of a water bound to a metal ion and the kobs produced by ferent divalent metal ions. That correlation has been inter- ted in the metal hydroxide model (Fig. 4) as an effect on 5' concentration of the aqueous metal hydroxide, which then O N ves as a Brønsted base in the abstraction of the proton from i 2Ј-oxygen. We have argued (12) that this logic is flawed, X-ray (active) O ause the metal hydroxide complexes4' formed with metal s with lower pKa values are weaker bases and, therefore, O O H uld be less able to abstract3' 2Ј-OH proton, despite their the B(-) ater concentration. This conclusion is supported by the data P sented in Fig. 3 because the pKa of the 2Ј-OH is two or more O Mg 2+ a units higher than those of any of the aqueous metal ions O R N died, making the metal hydroxide poorly suited to the task i+1 has O deprotonating the 2Ј-OH. It 5' been convincingly shown S O 2' O t proton transfer does 3' occur in the rate-determining not 2+ H O 4' R AH(+) Mg p of the ribozyme cleavage reaction (30). The observed pH pendence and the correlation between the pKa values of the ueous metal ions and kobs must, therefore, reflect the effects Mg2+/La3+ La3+/La3+ experimental: Pontius et al., 1997; Lott et al., 1998 Mg2+/Mg2+ theoretical: Boero et al., 2005; Leclerc & Karplus, 2006
- 14. Two-Metal Ion Models X-ray (active) 20Å A Specific Metal Ion in the Hammerhead Ribozyme 26823 longest time courses (48 –96 h). Each phase of the time course was 10-fold faster at pH 7.5 than at pH 6.5, as expected if each process were limited by the chemical step (15). Finally, purification of this phosphorothioate-substituted HH16 by anion exchange HPLC (8) re- sulted in partial separation of ribozyme forms such that the two phases had identical rate constants to those observed in the racemic mixture but different relative amplitudes (one fraction gave 0.8 of the fast component and 0.2 of the slow, whereas a second fraction gave 0.2 of the fast and 0.8 of the slow). Rates and relative amplitudes of the two phases for reactions in 10 mM Mg2⌅ did not change upon addition of 0.2 mM EDTA or 2 mM dithiothreitol to the reaction mixture, suggesting that neither kinetic process depended on the presence of contaminating metal ions. In reactions with added Cd2⌅, the concentration of EDTA carried over from the ribozyme and substrate stocks was ⌃15 nM. experimental: RESULTS Peracchi et al., 1997 We have used two different hammerhead ribozyme con- structs, HH⇥1 and HH16 (Scheme 1), in testing the role and
- 15. Reaction Path Modeling Reaction Path Following: B3LYP/6-31+G(d,p)//HF/3-21+G(d)
- 16. Contribution of Metals to Catalysis Relative Free Energy (kcal/mol) 30 exp ΔG = 20.1 kcal/mol 25 22.9 kcal/mol 20.8 kcal/mol 20 no metal 15 19.3 kcal/mol 1 metal 10 2 metals 5 dianionic mechanism 0 -5 -10 -15 -20 B3LYP/6-31+G(d,p) Lopez et al., 2006 B3LYP/6-311+G(2d,2p)//B3LYP/6-31G(d,p) Torres et al., 2003 -25 B3LYP/6-31+G(d,p)//HF/3-21+G(d) Leclerc & Karplus, 2006 -30 I II III IV V VI VII VIII IX Reaction Coordinate
- 17. ‘Ion Atmosphere’ Model No Metalloenzyme 5' O Ni O 4' 3' O O 2' H P B(-) O 5' OR Ni+1 OS O2' O 3' AH(+) H O R4' Murray et al., Chem. & Biol., 1998 Curtis & Bartel, RNA, 2001 O’Rear et al., RNA, 2001
- 18. Minimum and Full-Length HH ribozymes Wang et al., Biochem., 1999 Khvorova et al., Nat. Struct. Biol., 2003 de la Peña et al., EMBO J., 2003 Canny et al., JACS, 2004
- 19. A Nucleobase Catalyst X-ray (active) 5' O- H O C17 O4' H O6 N7 3' O O 2' H P N1 N R O 5' OR G12 OS N H2N H H O H O G8 2' O O 5' O experimental: Chi et al., 2008 theoretical: Lee et al., 2008
- 20. A Nucleobase Catalyst X-ray (active) 5' O- H O C17 O4' H O6 N7 3' O O 2' H P N1 N R O 5' OR G12 OS N H2N H H O H O G8 2' O O 5' O experimental: Chi et al., 2008 theoretical: Lee et al., 2008
- 21. A Nucleobase Catalyst X-ray (active) 5' O- H O C17 O4' H 4' O6 N7 3' O O 2' H P N1 N R O 5' OR G12 OS N H2N H H O H O G8 2' Mg2+ O O 5' O experimental: Chi et al., 2008 theoretical: Lee et al., 2008
- 22. Metal Ions / Nucleobases as Catalysts 5' 5' Ni O Ni O O4' O4' 3' O O 2' H 3' O O 2' H AH(+) P B(-) P O 5' OR O OR Ni+1 Ni+1 RO(-)H OS 5' OS O 2' O 3' AH(+) O 2' O 3' H O R4' H O R external nucleophile 4' internal nucleophile HDV, Hairpin, group-I, group-II introns Hammerhead, etc Fedor & Williamson, Nat. Rev. Mol. Cell Biol., 2005
- 23. Catalytic Strategies in Self- Cleaving Ribozymes 5' O Ni O4' Hairpin Ribozyme 3' O O 2' H P B(-) -O 5' O OR N Ni+1 OS O2' O 3' AH(+) N N H O R N R 4' N NH2 H2N G-8 N N H R N A-38 Rupert & Ferré d’Amaré, Nature, 2001
- 24. Catalytic Strategies in Self- Cleaving Ribozymes 5' O Ni O4' Hairpin Ribozyme 3' O O 2' H P B(-) O 5' O OR N Ni+1 OS O2' O 3' AH(+) H N N H O R N R 4' NH2 H2N N G-8 N N R N Salter et al., Biochem., 2006 A-38 Nam et al., RNA, 2008
- 25. Catalytic Strategies in Self- Cleaving Ribozymes 5' O Ni O4' HDV Ribozyme 3' O O 2' H P B(-) H2N O 5' OR Ni+1 OS O2' O 3' AH(+) N C-75 H O R N 4' R O OH2 2+ Mg Perrotta et al., NAR, 1999
- 26. Catalytic Strategies in Self- Cleaving Ribozymes 5' O Ni O4' HDV Ribozyme 3' O O 2' H P B(-) -HO O 5' OR Ni+1 OS Mg2+ O2' O 3' AH(+) H O R4' NH2 C-75 N H N R O Nakano & Bevilacqua, JACS, 2001 Liu et al., J. Phys. Chem., 2007
- 27. Metal Catalysts in the Hammerhead Ribozymes ? 5' O Ni O O- H 4' H 3' O O 2' H B(-) O6 P N7 O OR Mg2+ Ni+1 OS N1 N 5' G12 O2' O 3' N H O R AH(+) et al. 4' Osborne H2N e Scheme 1 n e r n d + U al z 2 Osborne et al., Biochem., 2009
- 28. Metal Binding in Self- Cleaving/Splicing Ribozymes A Hammerhead B HDV C-site C-site bridging-site bridging-site Hairpin Group-I C D Intron
- 29. unable to rescue activity for the A13 or A14 phosphoro- high negative potentia Metal Binding Sites in the thioate substitutions (Ruffner & Uhlenbeck, 1990; Knoll also modeled metal b et al+, 1997; Peracchi et al+, 1997; Scott, 1997)+ The A9 stead of the metal inte phosphate is part of a metal-binding site observed in posed here (Fig+ 4), the the original X-ray structure of the hammerhead (Pley with the N1 of G8 + We Hammerhead Ribozyme ? et al+, 1994), where a Mn 2ϩ ion is ligated by the pro-R P Brownian-dynamics sim FIGU dem the h high ing s ture The meta phat to m phat gand resid colo illust and Chartrand et al., RNA, 1997 Hansen et al., RNA, 2008
- 30. Metal Catalysts in the 2’OH activation ? O4' O4' < OH- 2' 2' 3'O O H OH- 3'O O H OH2 P Mg2+(VI) P Mg2+(VI) H 3C O OR H3C O OR 5' OS 5' OS General Base Lewis Acid Zdenek et al., J. Phys. Chem., 2011
- 31. Metal Catalysts in the 2’OH activation ? H H O4' O O 4' H2N H 2N N H O 2' H O 2' H < 3' O 3' O N N P N P 5' OR N 5' OR N H3C O H H3C O O OS H N OS O H N Mg2+(VI) H O H OH O4' O N 3' O O 2' H P N N H3C O 5' OR H OS N H2N Zdenek et al., J. Phys. Chem., 2011
- 32. General Acid/Base Catalysis in RNA cleavage 5' RNase A Ni O O4' His12 3' O O 2' H 5' P B(-) N NH O OR Ni+1 OS O 2' O 3' AH(+) +H N Lys41 O R 3 H 4' Phe120-NH- His119 N H HN Raines, Chem. Rev., 1998
- 33. Cooperative Models in Self-Cleaving ? Ni RNA5' O 4' H B(-) O O M/H-R 2' O 3' P O O Ni+1 O 5' O R-H/M AH(+) O OH RNA3'
- 34. Cooperative Models in Self-Cleaving ? Mg2+ O- N N C17 -O N R N N C17 RNA5' O 4' H R G-12 N RNA5' O 4' H N N O O H2N G-12 2' O O O 2' Mg2+ NH2 3' P O O 3' P O O O N1.1 Mg2+ O Mg2+ O N1.1 5' O 5' O O H O H 2' O OH 2' O OH G RNA3' G O RNA3' O O O G-8 RNA3' G-8 RNA3' O O RNA5' Leclerc, Molecules, 2010 RNA5'
- 35. Metal Ions back in the and DeRose 2000; Boots et al. 2008). Moderate rates of catalysis can also be achieved in molar concentrations of monovalent cations, an important property that helped to uncover the critical roles of nucleobases in the HHRz re- Hammerhead Catalysis action mechanism (Murray et al. 1998; O’Rear et al. 2001; Bevilacqua et al. 2004). At physiological ionic strengths, the HHRz requires divalent ions for appreciable rates of catal- 11 - Published by Cold Spring Harbor Laboratory Press divalent ysis; therefore, it is reasonable to assume that the metal-dependent channel is the primary mode of catalysis in nature (Khvorova et al. 2003). The HHRz was studied for years in its simplest active form, as three short helices meeting at a junction of con- served nucleotides that form the active site of the ribozyme (for review, see Blount and Uhlenbeck 2005). Studies using this ‘‘truncated’’ form of the HHRz (trHHRz) led to a model of catalysis in which a catalytic metal in the P9/ G10.1 site coordinates the pro-R oxygen of the scissile phosphate, presumably to stabilize the negative charge of the phosphorane transition state (Peracchi et al. 1997; Wang et al. 1999). Based on detailed metal-rescue exper- iments, Wang et al. (1999) predicted that the metal ion coordinates to the P9/G10.1 site in the ground state and bridges to the scissile phosphate in the transition state of the trHHRz reaction. A ground state that is very different from the transition state is consistent with structural studies of the truncated HHRz, which in general did not show catalytically relevant atoms within appropriate dis- tances of the active site (Blount and Uhlenbeck 2005). In ˚ these structures, the P9/G10.1 metal ion site is z20 A away from its predicted ligand during catalysis, the pro-R oxygen FIGURE 1. (A) Secondary structure of the modified Schistosoma Ward &DeRose,mansoni HHRz (MSL1L2) (Osborne et al.S. mansoni in these (2OEU) of the scissile phosphate (Pley et al. 1994; Scott et al. 1995). RNA, 2012 active site of the 2005) used HHRz studies. (B) Crystallographic
- 36. Metal Ions/Nucleobase Catalysts in the RNA World ES*≠ Mg2+ (Mg2+ + nucleobases) ES≠ Mg2+ (Mg2++ nucleobases) ES* ES non-enzymatic catalysis metal ion catalysis metal+nucleobase catalysis EP
- 37. Acknowledgments • Zdenek Chval (University of South Bohemia, CK) • Daniela Chvalová (University of South Bohemia, CK) • Xavier Lopez (Euskal Herriko Unibertsitatea, SP) • Annick Dejaegere (ESBS Strasbourg, France) • Darrin M. York (Rutgers University, USA) • Martin Karplus (Harvard University, USA)
- 38. Thank you ! G-12 C17 O2’ O3’ O2’ O5’ G-8 N 1.1