Hema Chapter 24_Hemostasisnnnnnnnnnnnnnnnn (2).ppt
- 1. Chapter 24 Hemostasis and disorders of coagulation
- 2. Objectives At the end of this chapter, the student will be able to: Describe normal and abnormal hemostasis Discuss how the components of normal hemostasis interact with each other to bring about normal blood flow with in the vascular system Explain the intrinsic and extrinsic pathways of blood coagulation Discuss the normal control of the clotting process and the fibrinolytic system
- 3. Objective cont’d State the principles of the different tests of the bleeding disorders Perform the different tests of the bleeding disorders Indicate the normal values of the different tests of the bleeding disorders
- 4. outline Introduction to hemostasis Components of coagulation Vascular system Platelets Coagulation factors Fibrinolysis Bleeding and coagulation disorders Laboratory diagnosis of Bleeding and Coagulation Disorders Bleeding time test Whole blood coagulation time test Clot retraction time Prothrombin time test with INR Partial thromboplastin time Thrombin time Fibrinogen Assay D-Dimer
- 5. 1 Introduction Hemostasis (Haima= blood and stasis=arrest) is a complex process which continually ensures prevention of spontaneous blood loss is the arrest of bleeding stops hemorrhage caused by damage to the vascular system. is initiated by vascular injury and culminates in the formation of a firm platelet-fibrin barrier that prevents the escape of blood from the damaged vessel
- 6. Definition… Hemostasis is defined as the process that maintains the flowing blood in a fluid state and confined to the circulatory system. •The hemostatic mechanism is not a single biological pathway, but the product of the complex interactions of a number of distinct systems: vascular system; blood platelets; blood coagulation system; fibrinolytic system; inhibitors of the above systems.
- 7. COMPONENTS OF COAGULATION The vital physiological function involves three components: vessels and vascular coagulation factors thrombotic: platelets and platelet coagulation factors humoral: plasma factors, coagulation and fibrinolysis activators and inhibitors.
- 8. phases Generally, this is described as a three –phase process division is arbitrary since the various phenomena are strongly interrelated: I . primary hemostasis II. secondary hemostasis-coagulation III. tertiary hemostasis-fibrinolysis
- 9. Phases cont’d I. Primary hemostasis involves the blood vessels ( parietal or vascular vasoconstriction phase and release of tissue or exogenous factors) and the thrombocytes (platelate or endothelial – thrombocyte phase, platelate aggregation and release of platelate factors). After 3 to 5 minutes , blood flow is arrested with the formation of a platelate plug.
- 10. Phases cont’d II. Coagulation involves plasma coagulation factors (plasma phase) platelet factor 3 provides for definitive hemostasis takes 5 to 10 minutes by formationof fibrin - stable clot reinforces the platelet plug. III Fibrinolysis essential final step in any hemostasis mechanism, enabling in 48 to 72 hours, and a return to normal by destroying fibrin and healing the injured vessel.
- 11. FORMATION OF A STABLE PLUG
- 12. I. Primary hemostasis Is a result of a three –way interaction between: the vascular wall the platelets plasma glycoprotein Fibrenogen and vWF Triggered by small injuries to blood vessels the plasma glycoprotein factors desquamation / damaging of epithelial cells in pinpricks
- 13. The vascular system The Blood vessel wall first line of defense for normal hemostasis lined with endothelial cells and synthesize von Willebrand factor (vWF) multimers vWF multimers are secreted into the circulation or onto the collagen-containing subendothelium. Following endothelial cell damage and subendothelial exposure, platelets bind to vWF multimers and collagen to initiate hemostasis. which form a tight selective membrane that keeps blood cells and plasma inside the vessel
- 14. The vascular system cont.d Endothelial cells also produce a fibrinolytic activator protective function to prevent blockage of blood vessels by clots Nerve and muscular tissue in the supporting sub endothelial (under the endothelium) tissue allow constriction of the vessel when injured (muscle cells contract)
- 15. The vascular system cont.d
- 16. The vascular system cont.d The blood vessels allowing the passage of gases nutrients selected cells to enter or leave the system In the normal state, the endothelial cells produce a substance called prostacyclin & other substances inhibit platelet function (cause disaggregation of platelets).
- 17. The vascular system cont.d Vasoconstriction: Constriction of small vessels such as arterioles, venules, capillaries slows or stops blood flow Serotonin Thromboxane A2 is a powerful vasoconstrictor and platelet aggregating agent
- 18. Vasoconstriction &Blood Flow Vasoconstriction creates stasis facilitating PLT activation and promoting secondary hemostasis. Blood flow controls hemostasis by diluting and removing activated factors. Graphic accessed at URL http://www.walgreens.com/library/contents.jsp?docid=8983&doctype=2, 2008.
- 19. The vascular system cont.d Slowed bleeding more effective platelet contact activation adhesion of platelets to the exposed subendothelial tissue wound sealed and vascular lumen narrows closes and blood flow to the injured site minimized Note: this effect is temporary lasting up to 20 seconds needs to be supplemented by platelets and blood coagulation factors
- 20. Platelets Platelets are anucleated, cytoplasmic fragments of the megakarocyte mother cell 4 stages of development: Megakaryoblast, Promegakarocyte, Megakaryocyte, Thrombocyte Each megakaryocyte produces 2000-4000 platelets
- 21. Platelets cont’d Young platelets are larger & less dense than older platelets. also metabolically active and more effective in hemostasis Platelet turn over rate equals 35,000 4,300 per L each day Size 2-20 fL; 2-4 m in diameter, colorless, with Wright’s stain they stain blue with pink granules
- 23. Platelets cont’d Life span ~9 days (7-10 days) Production regulated by thrombopoietin (plus others) Normally 2/3 of the platelets released from the bone marrow stay in the circulation; the remaining sequestered in a splenic pool that is freely exchangeable with circulating platelets
- 24. Platelets cont’d Function of platelets Maintain the functional integrity of the endothelial surface Initially arrest bleeding by forming temporary hemostatic platelet plug Provide phospholipids (Platelet factor 3) acts as a catalytic surface for initiation of the coagulation process When there is an injury platelets undergo the following actions: Adhesion Aggregation Shape change Secretions
- 25. Adhesion It is the binding of platelet to non platelet surface: sub endothelial collagen involves changes from a disc shape to a slightly broader, plate like form to increase surface area a number of plasma proteins are required for normal platelet adhesion. fibronectin and von Willebrand factor (vWF vWF is the largest component of factor VIII secreted by platelets and by vascular endothelial cells. Thrombin also stimulates platelet adhesion
- 26. Adhesion cont’d Collagen – vWF –Platelet Bridge physical distance between platelate and sub endothelial collagen Increase bond that seal platelet to the vessel wall reversible
- 27. Release reaction(secretions) It is release of contents of the granules of platelet Primarily ADP stimulates aggregation Cathecolamine (especially epinephrine) and serotoni enhance vasoconstriction Platelets contain 3 types of secretary granules: Lysosome containing acid hydrolyses α-granules containing platelate specific proteins (Plt factor 4, β- thromboglobulin, as well as other proteins such as Platelet derived growth factor and coagulation proteins found in plasma (fibrinogen & von Willebrand’s factor) -granules containing ATP, ADP, Calcium & serotonin
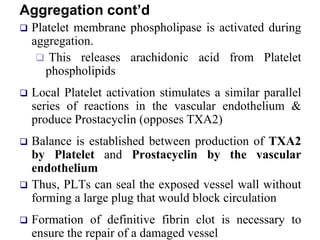
- 29. Aggregation Platelet-Platelet interactions Triggered by ADP Need FIBRINOGEN to bridge platelate-to- platelate distance and encourage platelate plug Platelate fill the open space to form a plug Platelates shed membranes rich in phospholipid (appearance of PLT factor 3 on the PLT membrane) this happens during PLT plug formation serves as a catalytic site for the coagulation proteins helps initiation of the coagulation mechanism Aggregation is also a response to thrombin and thromboxane
- 31. Aggregation cont’d Platelet membrane phospholipase is activated during aggregation. This releases arachidonic acid from Platelet phospholipids Local Platelet activation stimulates a similar parallel series of reactions in the vascular endothelium & produce Prostacyclin (opposes TXA2) Balance is established between production of TXA2 by Platelet and Prostacyclin by the vascular endothelium Thus, PLTs can seal the exposed vessel wall without forming a large plug that would block circulation Formation of definitive fibrin clot is necessary to ensure the repair of a damaged vessel
- 33. 2. Secondary hemostasis (coagulation) In the coagulation or plasma phase , blood changes from the fluid state to the gelled state , a result of the transformation of a soluble protein , fibrinogen , into an insoluble protein , fibrin . This forms the network around which the clot will be formed. This change in state corresponds to a cascade of enzyme activity whose first step have the function of amplifying the entire process of fibrin formation. This cascade requires a large number of protein factors , most of which are present as pro enzymes and which are transformed by partial proteolysis to active forms. Procoagulants are the enzymes ( zymogens), substrates and Co - factors
- 34. Enzymes( Serine Proteases) Hydrolyze peptide bonds Synthesised as inactive zymogen –cleaved at sites by another proteases Activation is localized ( at sites of injury)
- 35. Cont.. Factor I (Fibrinogen) Large, stable globulin protein (mol wt 341,000) Is the precursor of fibrin, which forms the resulting clot When fibrinogen is exposed to thrombin, two peptides split from the fibrinogen molecule, leaving a fibrin monomer. The monomers aggregate together to form the final polymerized fibrin clot product Factor II (Prothrombin) Is a stable protein (mol wt 63,000) In the presence of ionized calcium, prothrombin is converted to thrombin by the enzymatic action of thromboplastin from both extrinsic and intrinsic sources
- 36. Cont.. Factor IIa (Thrombin) Has a half-life of almost 3 days with 70% consumption during clotting Thrombin (mol wt 40,000) is the activated form of prothrombin, which is normally found as an inert precursor in the circulation This proteolytic enzyme, which interacts with fibrinogen, is also a potent platelet-aggregating substance A unit of thrombin will coagulate 1 ml of a standard fibrinogen solution in 15 sec at 28oC
- 37. Cont.. Tissue thromboplastin Tissue thromboplastin is the term given to any non- plasma substance containing lipoprotein complex from tissues. These tissues can be from the brain, lung, vascular endothelium, liver, placenta, or kidneys; these tissue types are capable of converting prothrombin to thrombin Ionized calcium (formerly factor IV) The term ionized calcium has replaced the term factor IV Necessary for the activation of thromboplastin, and for conversion of prothrombin to thrombin. Ionized calcium is the physiologically active form of calcium in the human body and only small amounts are needed for blood coagulation. A calcium deficiency would not be expressed as a coagulation dysfunction, except in cases of massive transfusion
- 38. Cont.. Factor V (Proaccelerin) Factor V is an extremely labile globulin protein. It deteriorates rapidly, having a half-life of 16 hours. Factor V is consumed in the clotting process and is essential to the later stages of thromboplastin formation Factor VII (proconvertin) Factor VII, a beta globulin, is not an essential component of the intrinsic thromboplastin-generating mechanism. It is not destroyed or consumed in clotting and is found in both plasma and serum, even in serum left at room temperature for up to 3 days. The action of factor VII acceleration of the production of thrombin from prothrombin. This factor is reduced by vitamin K antagonists.
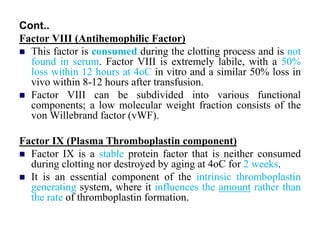
- 39. Cont.. Factor VIII (Antihemophilic Factor) This factor is consumed during the clotting process and is not found in serum. Factor VIII is extremely labile, with a 50% loss within 12 hours at 4oC in vitro and a similar 50% loss in vivo within 8-12 hours after transfusion. Factor VIII can be subdivided into various functional components; a low molecular weight fraction consists of the von Willebrand factor (vWF). Factor IX (Plasma Thromboplastin component) Factor IX is a stable protein factor that is neither consumed during clotting nor destroyed by aging at 4oC for 2 weeks. It is an essential component of the intrinsic thromboplastin generating system, where it influences the amount rather than the rate of thromboplastin formation.
- 40. Cont.. Factor X (Stuart Factor) This -globulin is a relatively stable factor that is not consumed during clotting. Together with factor V, factor X in the presence of calcium ion forms the final common pathway through which the products of both the extrinsic and intrinsic thromboplastin-generating systems merge to form the ultimate thromboplastin that converts prothrombin to thrombin. The activity of factor X appears to be related to factor VII Factor XI (Plasma Thromboplastin Antecedent) Factor XI, a -globulin, can be found in serum because it is partially consumed during the clotting process. These factor is essential to the intrinsic thromboplastin- generating mechanism.
- 41. Cont.. Factor XII (Hageman factor) Factor XII is a stable factor that is not consumed during the coagulation process. Adsorption of factor XII and kininogen (with bound prekallikrein and factor XI) to negatively charged surfaces such as glass or subendothelium (Collagen) exposed by blood vessel injury initiates the intrinsic coagulation pathway. Surface absorption alters and partially activates factor XII to factor XIIa by exposing an active enzyme (protease) site. Because of a feedback mechanism, Kallikrien (activated Fletcher factor) cleaves partially activated factor XIIa molecules adsorbed onto the subendothelium to produce a more kinetically effective form of XIIa. Factor XIII (Fibrin-Stabilizing Factor) Fibrin-stabilizing factor in the presence of ionized calcium produces a stabilized fibrin clot
- 42. Cont.. The clotting mechanism responsible for the formation of fibrin involves a cascade of reactions in which inactive enzymes (zymogens) are activated, and the activated enzymes in turn activate other inactive enzymes. Plasma coagulation factors have various names but an internationally standardized nomenclature system is using Roman numeral designations. A lower case “a” indicates the active factor (e.g. factor IXa) Roman numerals indicate inactive forms as they exist in the plasma except factors III & IV. They reflect order of discovery but not the sequence of reaction in the coagulation system
- 44. Cont.. Can be grouped as Fibrinogen group Thrombin sensitive I, V, VIII,XIII Prothrombin group - Vitamin-K dependent - II,VII,IX and X Contact group - XI, XII, PK, HMWK
- 45. Additional components: Ca 2+, v W F, Phospholipid Coagulation reaction occur on the surface of platelate phospholipid ( Platelate Factor 3) or endothelial cell membrane (phospholipid) Not in fluid phase Phospholipid is an assembly molecule
- 46. Cont.. Serine protease bind negatively charged phoapholipid surfacethrough positively charged Ca 2+ Ca involved in most reaction Ca bridge platelate factor with phospholipid, Phospholipid and Ca 2+ : used to overcome the influence of Inhibitory factors on plasma factors prevent diffusion of plasma factor in to the systemic circulation
- 47. Cont.. vWF is a large glycoprotein Platelate adhesion Transports procoagulant F VIII Synthesised in megakaryocytes and endothelial cells
- 48. Vitamin K in blood coagulation Coagulation factors II, VII, IX and X as well as protein C and protein S are dependent on vitamin K for their normal function • Vit K is found:
- 49. Cont.. They are synthesised in an inactive form that cannot bind Ca2+ only after posttranslational modification by γ carboxylation of glutamic acid residues can they bind
- 50. Cont.. In vit K deficiency ( in the presence of vit k antagonists:warfarin) there is no γ carboxylation → noncarboxylated forms of above proteins released into circulation These proteins cannot bind Ca2+ ions and thus cannot bind phospholipid surfaces, and hence cant participate in the coagulation reaction
- 52. Intrinsic system The initial reaction in this system is conversion of inactive factor XII to XIIa This activation is catalysed by high- molecular weight kininogen (HMW kininogen) and kallikrein Can be brought about in vitro by exposing the blood to electro-negatively charged wettable surfaces such as glass & collagen fibers Activation in vivo occurs when blood is exposed to collagen fibers underlying the endothelium in the vessels
- 53. Cont.. Contact factors do not have an in vivo procoagulant function. But responds to the negatively charged surfaces: non siliconized glass ( in a test tubes) In vivo activated by Valve prostheses Artificial implants in surgery Exposure to foreign substances: sub endothelial collagen
- 54. Cont.. Active factor XII then activates factor XI Active factor XI activates factor IX Activated factor IX forms a complex with factor VIII leading to activation of factor X; factor VIII itself needs activation by thrombin inorder to participate in the activation of factor X Phospholipids (PF-3) from aggregated PLTs & Ca++ are necessary for full activation of factor X
- 55. Cont.. Although intrinsic pathway is more complex & slower, it accounts for the majority of the coagulation activity in vivo Factor XII, Prekallikrein, HMW kininogen are referred to as the contact proteins, because their activation occurs on contact with an abnormal surface or (glass or kaolin)
- 56. Cont.. THROMBIN 1) cleaves Fibrinopeptides A and B from Fibrinogen 2)Amplifies co agulation mechanism- activates co factors V and VIII and F XI 3)activates F XIII Because of Multiple autocatalytic functions THROMBIN is considered the most important protease of the coagulation pathway
- 58. Extrinsic system (measured by PT) Coagulation is triggered by the exposure of tissue thromboplastin to plasma protein ( F-V II) -Tissue thromboplastin is not circulating in the blood but released when cells are damaged e.g. intravascular hemolysis, spontaneous abortion, traumatic head injury Concentrated sources of tissue thromboplastin (factor III) are: -RBC membranes,Platelets,Brain tissue,Placenta,Lung tissue
- 59. Cont.. The extrinsic system activates factor X rapidly since there are fewer reaction steps involved. Tissue factor forms a complex with factor VIIa and activates factor X to Xa
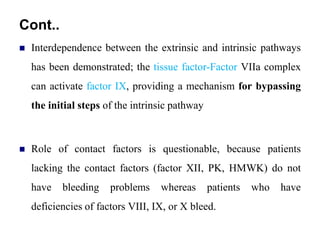
- 61. Cont.. Interdependence between the extrinsic and intrinsic pathways has been demonstrated; the tissue factor-Factor VIIa complex can activate factor IX, providing a mechanism for bypassing the initial steps of the intrinsic pathway Role of contact factors is questionable, because patients lacking the contact factors (factor XII, PK, HMWK) do not have bleeding problems whereas patients who have deficiencies of factors VIII, IX, or X bleed.
- 62. Common pathway Activated factor X, in association with cofactor on phospholipid surface and calcium,converts prothrombin to thrombin Thrombin converts fibrinogen to fibrin
- 63. Cont.. Currently proposed model is as follows: Following injury Tissue factor is expressed Complex formation with factor VIIa activates factors IX and X FXa binds FVa on Phospholipid surface in the presence of Ca 2+ This Xa-Va complex activates Prothrombin to Thrombin THROMBIN cleaves Fibrinopeptides A and B from plasma FIBRINOGEN causing the formation of Fibrin monomer ( soluble) then Fibrin polymer,which stabilized by the cross –linking action of F XIII to form Insoluble fibrin
- 64. Cont.. When small amounts of Xa are produced, tissue factor pathway inhibitor inhibits subsequent tissue factor activity (I.e., extrinsic pathway) Thrombin generated by the initial tissue factor activates factor XI to initiate the intrinsic coagulation and additional thrombin formation Thrombin generation is amplified by thrombin feedback activation of factors V and VIII
- 65. Cont.. Factor XII initiation is important when artificial surfaces are present, but not for in vivo coagulation So the current model explains why patients with deficiencies of factors VIII, IX, or XI bleed and why patients with contact factors deficiency do not
- 67. Fibrinolysis Lysis or dissolution of the clot (by the fibrinolytic system) Necessary for tissue repair to proceed and for normal circulation to resume For hemostasis to be effective, normal balance must exist between clot formation and removal Plasmin is the active component of the fibrinolytic system. It lyses fibrin and fibrinogen, with the production of fibrin degradation products
- 68. Cont.. In summary, following a vascular injury (injury of smaller vessels such as arteriole, venule, or capillary): Initially, rapid vasoconstriction reduces blood flow and promotes contact activation of platelets and coagulation factors In the second phase, platelets adhere immediately to the exposed sub endothelial connective tissue, particularly collagen. The aggregated platelets enhance sustained vasoconstriction by releasing thromboxane A2 and vasoactive amines, including serotonin and epinephrine In the third phase, coagulation is initiated through both intrinsic and extrinsic systems
- 69. Cont.. Finally, fibrinolysis occurs following the release of tissue plasminogen activators from the vascular wall. Fibrinolytic removal of excess hemostatic material is necessary to reestablish vascular integrity. Once tissue repairing is taking place, the clot dissolves gradually and the particulate matter is phagocytized by the mononuclear phagocytic system
- 70. Cont.. Normal control of the clotting process Equally important are mechanisms that prevent inappropriate activation of the cascade. Natural or innate inhibitors and anticoagulants circulate in the plasma, limiting the initiation and extent of fibrin formation. There are several protective mechanisms against thrombosis; the most important are: Removal of activated clotting factors by blood flow Inactivation of clotting factors by circulating inhibitors. The natural anticoagulant system in vivo includes:
- 71. The extent of the clot should be confined to the immediate surrounding area of the vascular lesion. Inhibitors to the fibrinogen activators are present in the blood to help control fibrinolysis
- 72. PHYSIOLOGICAL COAGULATION INHIBITORS 1. Antithrombin III -Is a serine protease inhibitor (SERPIN) - a glycoprotein of hepatic origin most powerful physiological coagulation inhibitors - markedly inhibiting thrombin (FIIa) - to a lesser degree on factors Xa, IXa, XIa, XIIa and kallicrein Requiers Heparin for effective anticoagulant activity
- 73. Proposed Mechanism of AT III- Heparin System Heparin Thrombin Antithrombin III Lysine sites Serine site Arginine site H Th H AT III AT III Th
- 74. Cont.. 2. Protein C-Protein S Protein C : vitamin K-dependent - synthesized in the liver activated by thrombin in the presence of Ca 2+ and a cofactor located on the surface of endothelial cells, thrombomodulin. Protein Ca (activated Protein C) inactivates the major proteins, factors Va and VIIIa. It requires as a co factor a phospholipids surface, Ca 2+ and is greatly enhanced by a plasma protein ,Protein S
- 75. Cont.. Protein S is -Vitamin K- dependent factor -synthesized in the liver -present in two forms in the plasma, a circulating form, and a form bound to the fourth component of complement (C4b) binding protein -only circulating Protein S is active as a cofactor of activated Protein C -Protein Ca activity is regulated by inhibitor ( PCI) Nb (Protein C is also active during fibrinolysis by neutralizing PAI
- 76. Cont.. 3. Heparin cofactor II (HC II) - a glycoprotein synthesized in the liver -unlike AT III, HC II is a very specific inhibitor and only neutralizes thrombin efficiently - this action is accelerated by heparin and dermatan - sulphate
- 77. Cont.. 4) Tissue factor pathway inhibitor (TFPI) - is emerging as the most important regulatory mechanism in vivo coagulation A) synthesised by endothelial cells and circulates in plasma bound to low density lipoproteins B) also present in platelets and bound to heparan sulphate at the endothelial surface C) TFPI inhibits coagulation by binding to factor Xa and TF:VIIa complex and inhibiting their proteolytic activity
- 78. FIBRINOLYSIS Fibrinolysis is the physiological process whereby fibrin is broken down by a specific enzyme, plasmin. When blood coagulation is activated ,it maintains hemostatic balance by dissolving the fibrin deposits which could occur spontaneously in the circulation and re open thrombosed blood vessel Plasmin is a result of activation of plasminogen.The activation process can have several pathways:
- 79. Cont.. (i) - tissue plasminogen activator (t-PA) - is synthesized in the endothelial cells and related in large quantities by various stimuli : venous stasis, acidosis, stress, physical exercise, - t-PA has a great affinity to fibrin which it binds to rapidly ,thus enabling plasminogen to be transformed in to plasmin at the fibrin clot, carrying out fibrinolysis in situ. -The t –PA bound to the fibrin clot is protected by the action of its inhibitors t-PA is found in fairly large quantities in organs such as the uterus, the prostate and the lungs
- 80. Cont.. (ii)-the urokinase – This activator has been detected in plasma and circulates in a zymogen form D –Dimers are specific products of fibrin degradation (4 main products called X, Y, D, E fragments). These fragments act as strong anticoagulants as its specificity is low , plasmin can attack other substrates such as anti hemophilic factor A ( F VIII), PRO ACCELERIN ( FV), etc
- 81. PHYSIOLOGICAL INHIBITOR OF THE FIBRYNOLYTIC SYSTEM As in coagulation, fibrynolysis is confined to the clot surface and controlled by inhibitors Their target is : -either the activation system The strongest inhibitor is the Plasminogen Activator Inhibitor (PAI). PAI inhibits t-PA and u-PA or plasmin whose principal inhibitor is alpha 2- antiplasmin
- 82. Bleeding disorder 1. Bleeding and Coagulation disorders Vascular defects Platelet defects Coagulation factors Vascular defects Inability to contract after injury Causes include:- Ascorbic acid (vit C) deficiency Inflammation Certain toxins Aging and Congenital defects e.g. hereditary hemorrhagic telangiectasia In these conditions, bleeding in to the skin produces ecchymoses called “vascular purpura”
- 85. Platelet defects Quantitative and qualitative defect Thrombocytopenia Decreased production Hypoplasia Marrow replacement by tumor or malignant cells Immune damage from toxins, drugs, bacterial & viral infections Idiopathic (ITP) Ineffective maturation e.g. in megaloblastic anemia Increased destruction or utilization Autoimmune antibodies DIC
- 86. Pooling of platelets by the spleen (without destroying PLTs) The spleen is responsible for destruction, anti-platelet antibody production and pooling Disorders of PLT function (qualitative defect) Acquired or inherited Acquired Platelet disorders Many drugs e.g. asprin, other non-steroidal anti- inflammatory drugs Hereditory Platelet disorders Thrombasthenia defect in primary platelet aggregation
- 87. Coagulation factor defect and inhibitors Coagulation factor defect or abnormal function Factor deficiencies The most important congenital deficiency is Factor VIII deficiency: Called Hemphilia A Graded as severe, moderate, and mild depending on the coagulant activity of Factor VIII Inherited as sex-linked recessive manner and occurs exclusively in males
- 88. von Willebrand’s disease: Due to deficiency of vWF Characterized by defects in platelet adhesion Factor IX deficiency: Called Hemophilia B Sex linked recessive Occurs less frequently and milder in its clinical presentation than factor VIII deficiency (Hemopilia A)
- 89. Abnormal coagulation factors function Abnormality in function is seen in vit K deficiency. The binding of Ca++ to factors II, VII, IX, X is required for normal clotting. Without the attached Ca++, these fators will not bind to phospholipids and rate of factor activation will be sharply decreased. To bind Ca++ they need gamacarboxylation with vit K (to make II, VII, IX & X functional) Vit K dependant factors are: Factors II, VII, IX, X, Protein C & protein S
- 90. Consumption of coagulation factors e.g. DIC acquired coagulation defect secondary to other pathologic processes which results in accelerated consumption of platelets and several coagulation factors, particularly fibrinogen Inhibitors of coagulation e.g Lupus anticoagulant in patients with SLE and other related disorders. Lupus anticoagulant interferes with the phospholipid portion of Factor Xa-V-Ca++-Plt phospholipids complex.
- 91. Disturbance of the balance between promotors and inhibitors of coagulation due to: Various disorders including bacterial, viral, rickettsial, infections Complication of pregnancy AML Tissue damage (shock, heat strock, burns) Hemolytic transfusion reactions Venome snake bites
- 92. 2. Laboaratory investigation of bleeding and coagulation disorders Bleeding Time Coagulation time Platelet count Clot retraction time Prothrombin time (PT) Accctivated Partial Thromboplastin Time (APTT) The Thrombin time Fibrinogen quantitative assays specific factor assays are commonly used to assess coagulation factors. preoperative screening tests usually include a bleeding time, platelet count, PT & APTT
- 93. Bleeding time: the time required for a small standardized wound cut to stop bleeding a measure of vascular integrity and platelet function prolonged in: shortage of platelets (Plt<50,000 cells/L)(Thrombocytopenia) inadequate function of platelets von Willebrand’s disease poor retractability of capillaries (e.g. scurvy-vit C deficiency) deficiency of plasma factors
- 94. Different methods: Duke method & Ivy method (common ones), Mielke (9 mm long, 1mm deep), Template, Simplate (5mm long, 1mm deep) Duke method: oldest method, which is performed by puncturing the ear lobe using a sterile lancet, recording the time, blotting the blood every 30 seconds until bleeding ceases, and recording the time. Blotting is done without allowing the filter paper to touch the wound
- 95. Cont.. Time between the puncture and the cessation of bleeding is the bleeding time NR = 1-3 min (3-6 min boarder line) Drawback: impossible to standardize the depth of the incision; as a result not recommended
- 96. Cont.. Ivy method A blood pressure cuff is placed on the patient’s arm above the elbow, inflated & maintained at a constant pressure (40 mmHg) throughout the procedure. This is to standardize the pressure in the vascular system. Two or three standardized (3 mm) punctures of the forearm are made by holding the skin tightly i.e. by grasping the underside of the arm firmly. The length of time required for bleeding to stop is recorded. Report the average of the two results including method and normal values
- 97. Cont.. Note: wait for 30 sec after applying the sphygmomanometer and inflating it to 40 mm Hg to allow the capillary filling to equilibrate. Select an area in the lower arm 3-finger width below the bending in the elbow, with no hair and superficial veins. NR= 1-7 min; 7-11 min boarder line In general, if bleeding continues for more than 15 min, discontinue the procedure and report as >15 min. It is recommended to repeat the procedure on the other arm if bleeding time < 1min and >7 or 15 min, except in excessively prolonged tests.
- 98. Cont.. Sources of error No bleeding because of too gentle an incision Severe bleeding: superficial veins have probably been cut If filter paper touches the wound, a platelet aggregate might be removed resulting in prolonged bleeding Decrease bleeding time: Failure to cleanse the area Making superficial puncture
- 99. Cont.. Increase bleeding time: Puncturing a red flushed area Too deep puncture Applying pressure to the punctured area
- 100. Cont.. Coagulation time The time required for a measured amount of blood to clot under certain specified conditions Capillary blood methods Slide method (NR 2-6 min) Capillary tube (NR 2-6 min) Dale & Laidlaw (NR 1-3 min) Venous blood methods Lee & Wite method (NR 5-15 min) Howell method (NR 10-30 min) Silicone tube method (NR 20-60 min) Note: the report should always include method and normal range
- 101. Cont.. Slide method Puncturing the finger, recording the time, placing 3 drops of blood on a glass slide, drawing the point of the needle or lancet through the drops until fibrin threads appear & recording the time Capillary tube method Puncturing, recording the time, filling a capillary tube with blood, allowing 3 min to ellapse, breaking the capillary tubes at half min intervals until a span of fibrin is seen and recording the time
- 102. Cont.. Dale & laidlaw Puncturing, recording the time, allowing the blood to flow into a capillary tube, which contains a lead bead, immersing the capillary tube in 37oC water, tilting the tube until the lead bead is held firmly by fibrin threads, & recording the time Lee & White Drawing blood from a vein & noting the time the blood enters the syringe, or vacutainer tube, transferring the blood to 3 test tubes, tilting each test tube one after the other until coagulation takes place, & recording the time
- 103. Cont.. Howel method Coating a syringe with petroleum, drawing blood from a vein, recording the time the blood enters the syringe, transferring the blood to a test tube, tilting the tube until coagulation takes place, & recording the time Silicone tube method Coating a syringe with silicone to decrease the contact of blood with glass (because contact with glass promotes coagulation), drawing blood from a vein & noting the time the blood enters the siliconized syringe, transferring the blood to 2 siliconized test tubes, placing the tubes on 37 oC water bath, tilting the tubes until coagulation takes place & recording the time The Lee & White method is the most widely used method.
- 104. Cont.. Lee & White method In the past, it was used as a screening test to measure all intrinsic coagulation system & to monitor heparin therapy. However, it is time consuming, sensitive to only severe factor deficiencies & insensitive to high doses of heparin, and has poor reproducibility. Therefore, of limited use in today’s laboratory Procedure Label 3 clean 13 x 100 mm test tubes Draw 4 ml of blood, start the stopwatch as soon as blood enters the syringe Remove the needle & gently transfer about 1 ml of blood to each of the 3 test tubes (excessive agitation will hasten coagulation)
- 105. Cont.. Place the 3 test tubes in a 37oC water bath Allow 4-5 min to elapse & gently tilt test tube #1 to a 45 angle every 30 sec until the blood is completely clotted Repeat the process with the 2nd and 3rd test tubes & record the time Coagulation time is the time elapsed between the withdrawal of blood and completion of coagulation in test tube #3. Note: 3 test tubes are used because each successive tube is subjected to less tilting, & therefore, less agitation of the blood, and consequently a more accurate coagulation time. Since agitation and handling speed up coagulation, the coagulation time of test tube #3 is the reported result.
- 106. Cont.. Sources of error Coagulation hastened: Dirty test tubes Poor venipuncture can introduce tissue thromboplastin Excessive agitation during transfer Coagulation retarded: Temperature < 35 oC & > 45oC
- 107. Cont.. Diameter of the test tube; the smaller the diameter, the more rapid the clot formation is because the amount of foreign surface area (glass) to the amount of blood is increased. Therefore, all test tubes should be of the same diameter. Increased volume of blood per tube At completion of the Lee & White clotting time, it is suggested that 1 test tube remain in the 37oC water bath to be checked after 2 & 4 hours for clot retraction. Also the same tube may be allowed to remain in the water bath over night & checked the next day for clot lysis NR 5-15 min
- 108. Cont.. Standardization of Clotting time can be achieved when: The amount of blood is consistent Process occurs at specified temperature and Clotting occurs in the same physical environment
- 109. Cont. Platelet count (Thrombocyte count) Also involved in clot retraction Thrombocytosis In Polycythemia Vera: over active bone marrow resulting in increased production of WBC, RBC & Plt Idiopathic thrombocythemia (increased platelet number) CML Following splenectomy Sickle cell anemia: hyperactive bone marrow to produce more RBC, but increased WBC & Plts as well
- 110. Cont.. Thrombocytopenia Thrombocytopenia purpura (Idiopathic or secondary to other diseases) Aplastic anemia: characterized by pancytopenia Acute leukemia: decrease in Plt and RBC production Pernicious anemia: decreased production due to deficiency of vit B12 Sometimes following chemotherapy and radiation therapy Platelet count may be below or above NR values in certain normal conditions and activities e.g. below normal: before menstruation above normal: at high altitude and after severe exercise
- 111. Cont.. Platelets are difficult to count, small, disintegrate easily, and are hard to distinguish from dirt, readily adhere to each other (aggregation) and also become easily attached to any foreign body (adhesiveness). Platelet counts from finger or heel prick blood is less satisfactory and significantly lower than platelet counts obtained from venous blood. Significant number may probably be lost at the puncture site. When obtaining capillary blood, it is important that the platelet count should be obtained first
- 112. Platelet counts Blood is mixed with a diluent (1% ammonium oxalate) that causes hemolysis of red cells. A hemocytometer is filled with the diluted sample and platelets are counted under the microscope Note: Amonium oxalate should be stored in refrigerator and always be filtered just before use to remove crystals and other debris, which may be mistaken for platelets. Platelet estimates (for QC purpose) In normal blood smear there are 8-20 Plts per field in the thin area (4-8 Plts/100 RBC; 30 Plts for every 500 RBC) One method of estimating platelets is to determine the average number of platelets per field, using 10 different fields, taking the average and multiply the result by 20,000 (comment as NR, decreased or increased) Never report an estimate; this is just for QC purpose
- 113. Procedure Venous EDTA blood recommended, not > 1 hr old because platelets disintegrate easily Pipet 0.38 ml of diluting fluid, Mix the blood Add 20 L blood and wash out pipet by drawing up the diluting fluid and expelling into the tube a few times Mix at least 10 min to ensure proper mixing and RBC lysis Blood diluted with 1% ammonium oxalate is stable for 8 hrs Technical tips The hemocytometer should be clean (ethyl alcohol & lint free cloth recommended) Shake gently, at least 2 min Allow 10-20 min for platelets to settle Count platelets in the central squares; there should be even distribution of cells in the counting chamber (there should be no platelet clump) Calculate the number of platelets and report with normal values
- 114. Cont.. Sources of error Capillary blood: platelets adhere to the wound; squeezing with heavy pressure may cause disintegration Ammonium oxalate shoud be refrigerated and must be discarded if there is evidence of bacterial contamination Presence of clumps: test should be repeated. Clumps are usually due to inadequate mixing or poor technique in obtaining blood sample Unfiltered diluting fluid: debris and dirt may be mistaken for platelets Dirty tubes, pipets, and counting chamber: Plts will stick to the dirt and falsely lower Plt count For QC purpose charge both sides of the hemocytometer Results should be double checked by examination of Plts on a Wright stained blood smear. If the count does not agree with the estimate, it should be repeated
- 115. Cont.. Clot retraction Time When blood coagulation is complete, the clot normally undergoes contraction, where serum is expressed from the clot, and the clot becomes denser (firm). Thrombosthenin, released by platelets is responsible for clot retraction. The time is affected by quantitative and qualitative defects in platelets. When the red cell count is high, degree of retraction decreased because of large volume of RBC in the clot, and vise versa. There are different methods. Some inspect the clot after 1, 2, 4 and 24 hours NR 2-4 hrs; Poor 4-24 hrs; >24 hrs reported as none
- 116. Cont.. Prothrombin time (PT) PT is the time required for plasma to clot after an optimal amount of tissue thromboplastin and calcium chloride have been added to trigger the coagulation process. It evaluates the generation of thrombin and the formation of fibrin via the extrinsic and common pathways
- 117. Cont.. Diagnostic significance It measures the functional activity of factors VII, X, and V, and factor II or I. Especially useful for initiation and monitoring of oral anticoagulant therapy to adjust the dose. Therefore, extreme care is needed. e.g. Warfarin is a vitamin K antagonist and interferes with the production of vit K dependent factors (factor II, VII, IX, and X) and Protein C & protein S. Protein C and S are natural anticoagulants. To diagnose deficiencies in the coagulation factors of the extrinsic system Useful for checking the synthesis performance of the liver in hepatic disease
- 118. Cont. Principle: The coagulation process is triggered by incubation of plasma with the optimal amount of thromboplastin and calcium, and the time required for the formation of a fibrin clot is measured in seconds. Prolonged in: Deficiency of one or more coagulation factors in the extrinsic pathway: i.e., factors VII, X, V, and II or I Vit K deficiency Certain liver diseases Circulating anticoagulants Anticoagulant therapy (e.g. Coumarin) DIC (disseminated intravascular coagulation)
- 119. Cont.. Quick’s one stage Prothrombin Time Specimen required: 9 parts blood + 1 part 3.8% sodium citrate. Avoid formation of foam. The sample should be centrifuged at 3000 rpm for 15 min as soon as possible with the plasma removed from the erythrocytes. Plasma may be stored several hours at 2-6oC prior to testing
- 120. Cont.. Quality control Control and patient plasma should be run in duplicate and the two results averaged to obtain the final value. Duplicates should agree within 2 sec. Report both patient and contol value; for patients on anticoagulant therapy, the control value is twice the normal. Normal values range from 10-13 sec. If the PT of the control plasma doesn’t lie within the specified values provided by the manufacturers, it indicates failure in equipment, reagent or techniques used and the test must be repeated
- 121. Cont.. Most common sources of error The 9:1 ratio of blood to sodium citrate should be precise Failure to follow directions in the manufacturers instruction (package insert) strictly while preparing patient plasma, control plasma, reconstituting reagents Use of dirty or wet test tubes, pipets etc to perform the test Test must be performed within 4 hrs of specimen collection (within 2 hrs is best) Mistakes in pipetting Hemolysis Timing, incubation temperature, contact activation influence the test
- 122. Cont.. International Normalized Ratio (INR) INR values preferable to the PT because different thromboplastin reagents have different sensitivities to warfarin induced changes in levels of clotting factors The INR corrects most of reagent differences, expressed as ISI ISI is the international sensitivity index of the thromboplastin reagent; it is a correction factor assigned by the manufacturer
- 123. Cont.. ISI values reported by manufacturers vary depending on the instrument used to perform the PT The PT, utilized to adjust the dose of oral anticoagulation, should be reported according to the INR and not the PT ratio or PT in seconds The INR is essentaially a “corrected” PT INR = PT patient ISI PT normal
- 124. Cont.. Activated Partial Thromboplastin Time (APTT) Major screening test for coagulation disorders in the intrinsic system Especially for sensitive detection of fatctors VIII and IX and the contact factors (except for platelets and factor XIII) Also a method of choice for monitoring heparin therapy
- 125. Cont.. Test principle: The plasma after centrifugation contains all intrinsic coagulation factors except Ca and platelets. Calcium and a substitute for Platelet factor III, (partial thromboplastin-Cephalin) and an activator such as kaolin, to ensure maximal activation, are added to the plasma. The time required for the plasma to clot is the APTT. The activator is added to ensure maximal activation.
- 126. Cont. When an activator is not added the test is called PTT and the amount of time required for normal plasma to clot is prolonged Normal plasma should be run each time a new reagent is opened The APTT assay reflects the activity of prekallikrein, HMWK, and factors XII, XI, IX, VIII, X, V, II, and I APTT may be prolonged due to a factor decrease or presence of circulating anticoagulants. The normal APTT is less than 35 seconds depending on the activator used
- 127. Interpretation The common causes of a prolonged APTT are as follows: Disseminated intravascular coagulation Liver disease Massive transfusion with plasma-depleted red blood cells Administration of or contamination with heparin or other anticoagulants A circulating anticoagulant (inhibitor) Deficiency of a coagulation factor other than factor VII
- 128. Prolonged APTT + Prolonged PT: Vitamin K deficiency Liver disease due to: -Malabsorption of vitamin K -Decreased synthesis of clotting factors - An acquired dysfibrinogenemia due to changes in the sialic acid content of the fibrinogen. Combined deficiency of clotting factors e.g. Factors V and VIII DIC - due to the consumption of clotting factors Massive blood transfusion leading to a dilutional coagulopathy
- 129. Cont.. Thrombin Time Determines the rate of thrombin induced cleavage of fibrinogen to fibrin monomers and the subsequent polymerization of fibrin polymers NR=<20 sec Prolonged When fibrinogen concentration is <100 mg/dL( Hypofibrinogenemia) Dysfibrynogenemia Afibrinogenemia: DIC,Liver disease Thrombin inhibitors or substances that interfere with fibrin formation (e.g heparin, fibrin degradation products)
- 130. Cont.. Fibrinogen levels Useful to detect deficiencies of fibrinogen and alterations in the conversion of fibrinogen to fibrin NR= 200-400mg/Dl May be decreased in liver disease or due to consumption of fibrinogen when there is accelerated intravascular clotting mainly affected by the concentration of Fibrinogen and the FDP level Elevated ;-in infection, Inflammation, Traumatic injury
- 131. Platelet Count PT APTT TT Fibrinogen Interpretation N N N N N Normal profile Factor XIII deficiency Mild VWD Qualitative platelet disorder Connective tissue problem e.g. Ehlers Danlos Mild coagulation factor deficiency N ↑ N N N FVII deficiency N N ↑ N N FVIII, FIX, FXI or FXII deficiency VWD - if the FVIII level is reduced Lupus anticoagulant [Occasionally a very strong lupus anticoagulant or a lupus anticoagulant that has anti-prothrombin activity can result in a prolonged PT. Similarly a lupus anticoagulant can also be associated with thrombocytopaenia.] Other contact factor deficiency N ↑ ↑ N N Common pathway' deficiency i.e. FII, FV or FX deficiency Multiple clotting factor deficiencies e.g. combined FV and FVIII deficiency Warfarin or vitamin K antagonist Vitamin K deficiency or a mutation within one of the genes encoding key enzymes involved in VK metabolism. Occasionally a very strong Lupus anticoagulant can cause these findings but it is unusual to see a prolongation of the PT with a LA due to the high concentration of PL used in the PT test. ↓ ↑ ↑ ↑ ↓ DIC Massive transfusion Liver disease ↓ N N N N Primary platelet problem e.g. ITP. The Mean Platelet Volume [MPV] can be helpful in establishing the causes of thrombocytopaenia. A raised MPV is often associated with increased peripheral destruction e.g. ITP - whereas a reduced MPV is often seen in association with bone marrow failure. Changes in the MPV are also seen in patients with various inherited platelet disorders e.g. Wiskott Aldrich syndrome
- 132. 35-year-old woman needs to have an ovarian cyst removed. Her mother has a history of bleeding after tooth extraction. The physician needs to determine if there is a bleeding disorder. The coagulation test results are as follows: PT 20.5 seconds (Reference range, 10.5 to 13.3) aPTT 32.1 seconds (Reference range, 28.7 to 35.5) Platelets 320,000/mm3 (Reference range, 150,000 to 400,000/mm3) Bleeding time 11 minutes What is impaired factor? A. V B. VII C. VIII D. IX
- 133. A 52-year-old woman needs to have tooth extraction. She had no pregnancy or delivery history. Her sister has a history of bleeding after minor surgery. The physician needs to determine if there is a bleeding disorder. The coagulation test results are as follows: PT 11.0 seconds aPTT 30.1 seconds Platelets 320,000/m m3 Bleeding time 13 minutes (Reference, 8 minutes) What is the most significant abnormal result in the coagulation panel? A. PT B. aPTT C. PLT D. BT
Editor's Notes
- #27: Adhesion































































































































![Platelet
Count
PT APTT TT Fibrinogen Interpretation
N N N N N Normal profile
Factor XIII deficiency
Mild VWD
Qualitative platelet disorder
Connective tissue problem e.g. Ehlers Danlos
Mild coagulation factor deficiency
N ↑ N N N FVII deficiency
N N ↑ N N FVIII, FIX, FXI or FXII deficiency
VWD - if the FVIII level is reduced
Lupus anticoagulant [Occasionally a very strong lupus anticoagulant or a lupus
anticoagulant that has anti-prothrombin activity can result in a prolonged PT.
Similarly a lupus anticoagulant can also be associated with thrombocytopaenia.]
Other contact factor deficiency
N ↑ ↑ N N Common pathway' deficiency i.e. FII, FV or FX deficiency
Multiple clotting factor deficiencies e.g. combined FV and FVIII deficiency
Warfarin or vitamin K antagonist
Vitamin K deficiency or a mutation within one of the genes encoding key
enzymes involved in VK metabolism.
Occasionally a very strong Lupus anticoagulant can cause these findings but it is
unusual to see a prolongation of the PT with a LA due to the high concentration
of PL used in the PT test.
↓ ↑ ↑ ↑ ↓ DIC
Massive transfusion
Liver disease
↓ N N N N Primary platelet problem e.g. ITP.
The Mean Platelet Volume [MPV] can be helpful in establishing the causes of
thrombocytopaenia.
A raised MPV is often associated with increased peripheral destruction e.g. ITP
- whereas a reduced MPV is often seen in association with bone marrow failure.
Changes in the MPV are also seen in patients with various inherited platelet
disorders e.g. Wiskott Aldrich syndrome](https://image.slidesharecdn.com/hemachapter24hemostasis2-240407192832-11467d4c/85/Hema-Chapter-24_Hemostasisnnnnnnnnnnnnnnnn-2-ppt-131-320.jpg)

