Structural Formula and Structural Model.ppt
- 1. Chapter 3 Chemical Compounds When atoms approach other in a chemical reaction, the electrons of the atoms interact to form chemical bonds. Compounds are substances composed of more than one element, chemically combined. E. g. HCl, H2 O, NH3 There are three fundamental kinds of chemical bonds between atoms-covalent bonds, ionic bonds and metallic bonds.
- 2. Types of Chemical Compounds and Their Formulas i) Molecular Compounds ~ A molecular compound is made up of discrete units called molecules, which typically consist of two or more of nonmetal atoms held together by covalent bonds. A covalent bond, the most common kind of chemical bond, results when two atoms share electrons. Even some elements exit as molecules rather than as atoms. Hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, iodine, sulfur and phosphorus all exist as molecules whose atoms held together by covalent bond. Therefore, we have to write them as H2 , N2 , O2 , F2 , Cl2 , Br2 , I2 , S8 and P4 when using any of these elements in a chemical equation.
- 3. Chemical Formulas A compound is represented by giving its chemical formula, a notation that uses atomic symbols with numerical subscripts to convey the relative proportion of atoms of different elements in the substance. H2O The two elements present two H atoms per formula unit Lack of subscript means one atom of O per formula unit E.g. CH2O The three elements present two H atoms per formula unit Lack of subscript means one atom of C per formula unit Lack of subscript means one atom of O per formula unit
- 4. Some Common Types of Formulas i) An empirical formula is the simplest formula for a compound; it shows the types of atoms present and their relative numbers. Compounds with different molecular formulas can have the same empirical formulas and such substances will have the same percentage composition. E.g. Acetic acid (C2 H4 O2 ), formaldehyde (CH2 O), and glucose (C6H12O6) all have the empirical formula CH2 O. Generally, empirical formula does not tell us much about a molecule. ii) A molecular formula is based on an actual molecule of a compound. It gives the exact number of different atoms of an element in a molecule. In some cases, the empirical formula and the molecular formula are identical E.g. formaldehyde CH2 O. In other cases, the molecular formula is a multiple of the empirical formula unit E.g. C6 H12 O6 = (CH2 O)6
- 5. Compound Molecula formula Empirical formula Ratio of atoms in compound Carbon dioxide CO2 CO2 1 carbon atom : two oxygen atoms formaldehyde CH2 O CH2 O 1 carbon atom : two hydrogen atoms : 1 oxygen atom Acetic acid C2 H4 O2 CH2 O 1 carbon atom : two hydrogen atoms : 1 oxygen atom Empirical and molecular formulas tell us the combining ratio of the atoms in the compound, but show nothing about how the atoms are attached to each other. There are other types of formula that will show the connectivity of atoms in a molecule.
- 6. Structure and Condensed Formulas iii) A structural formula shows the order in which atoms are bonded together in a molecule and by what types of bonds. The covalent bonds in the structure formula are represented by lines (). Each line represents one bond. iv) A condensed structural formula: a less cumbersome way of showing how the atoms are connected C H H O C O H H structural formula condensed formula CH3COOH
- 7. acetic acid CH2 O C2 H4 O2 name empirical formula molecular formula C H H O C O H H structural formula condensed formula CH3COOH
- 8. Molecular Models ball-and-stick type space-filling type To show three-dimensional structures of molecules is by structural models
- 9. Structural Models 1) ball-and stick-model, the centers of the bonded atoms are represented by small balls, and the bonds between atoms by sticks. Such model help us to visualize distances between the centers of atoms (bond length) and the geometrical shapes of molecules. 2) A space-filling model shows that the atoms in a molecule occupy space and that they are in actual contact with one another. This model is the most accurate representation of the size and shape of a molecule because it constructed to scale.
- 10. Ionic Compounds A ionic compound is made up of positive and negative ions (cation and anion) joined together by ionic bonds. A ionic bond result from a transfer of one or more electrons from one atom to another then joint together by electrostatic forces of attraction. For example, the arrangement of Na+ions and Cl-ions in a crystal of sodium chloride. Each Na+ ion is surrounded by six neighboring Cl- ions, and each Cl- ion is surrounded by six neighboring Na+ ions.
- 11. Formula unit of an ionic compound There is no discrete “molecule” of NaCl. Instead, the entire crystal is an ionic solid. The formula unit of an ionic compound is the smallest electrically neutral collection of ions. The ratio of atoms (ions) in the formula unit is the same as in the chemical formula Na + Cl Na + Cl + _ NaCl A chlorine atom A sodium atom A chlorine anion A sodium cation lose/ gain e-
- 12. Organic and Inorganic Compounds • Chemical compounds also can be classified as organic or inorganic. Organic compounds are those formed by carbon and hydrogen (hydrocarbon) or carbon and hydrogen together with oxygen, nitrogen, and a few other elements. • Inorganic compounds are compounds composed of elements other than carbon. Except a few simple compounds of carbon, including carbon monoxide, carbon dioxide, carbonates and cyanides are generally considered to be inorganic.
- 13. Formula Mass of Compounds i) Formula mass Formula mass(weight): is the mass of a formula unit in atomic mass unit. F.M.(or F. W.) = Sum of atomic mass of all the atoms in a formula unit of the compound, whether molecular or not. Molecular mass: is the mass of a molecule in atomic mass unit. M. M.(or M. W.) = Sum of atomic mass of all the atoms in a molecular. For a molecular compound, the formula unit an actual molecule and FM =MM.
- 14. E.g. Calculate the formula mass (weight) of each of the following (a) Sodium chloride FM NaCl = 1 atomic mass Na + 1 atomic mass Cl = 22.99 amu + 35.45amu = 58.44 amu (b) Fe(III) sulfate, Fe2 (SO4 )3 FM = 2(atomic mass Fe) + 3(atomic mass S)+ 12(atomic mass O) = 2(55.85amu) + 3(32.07amu) + 12(16.00amu) = 399.91amu
- 15. Mole of Compound A mole is an amount of substance that contains the same number of elementary entities (atoms, molecules or formula units) as the number of atoms in exactly 12g of carbon-12. (the quantity of a substance whose mass in gram is numerically equal to the formula mass of the substance). A mole of compound is an amount of compound containing Avorgadro’s number of formula units or molecules. The molar mass is the mass of one mole of compound. Formula Units Mole relate to grams by related by Avogadro's number Molar mass
- 16. For elements H2 , N2 , O2 , F2 , Cl2 , Br2 , I2 , S8 and P4 , we speak of atomic mass or molecular mass, and the molar mass can be expressed in two ways. E.g. atomic mass of hydrogen = 1.008 amu and a molecular mass = 2.016 amu; its molar mass can be expressed as 1.008g H / mol H or 2.016g H2 / mol H2 .
- 17. E. g. Both empirical and molecular formula of halothane is C2 HBrClF3 ; (a) What is its molecular mass and its molar mass? (b) How many F atoms are present per mole of halothane? From the formula, one mole of halothane contains two moles of C atoms, one mole each of H, Br and Cl atoms, and three moles of F atoms.
- 18. • E.g. How many H2O molecules are there in 1.00 L of water ? Density of water is 1.00 g/cm3
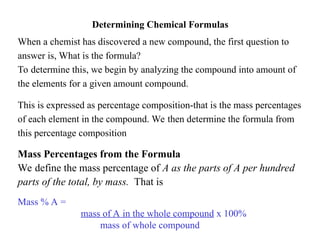
- 19. Determining Chemical Formulas When a chemist has discovered a new compound, the first question to answer is, What is the formula? To determine this, we begin by analyzing the compound into amount of the elements for a given amount compound. This is expressed as percentage composition-that is the mass percentages of each element in the compound. We then determine the formula from this percentage composition Mass Percentages from the Formula We define the mass percentage of A as the parts of A per hundred parts of the total, by mass. That is Mass % A = mass of A in the whole compound x 100% mass of whole compound
- 20. E.g. Calculate the percentage composition from formula (a) What is the mass percent composition of formaldehyde, CH2 O?
- 21. 1 Check the accuracy of the computations by ensuring that the percentages do total 100%. 2 Determine the percentages of all the elements but one. Obtain that one by difference (subtraction). The above example %O = 100.00% - % C -% H = 100.00% - 39.99% -6.73% = 53.28% (b) Calculating the mass of an element in a given mass of compound How Many grams of carbon are there in 83.5g of formaldehyde? use the percentage composition obtain in (a)
- 22. Establishing Empirical Formulas from the Experimentally Determined Percent Composition of Compounds Elemental Analysis: percentage of Carbon, Hydrogen, and Oxygen Percent composition establishes the relative proportion of the elements in a compound on a mass basis. A chemical formula requires these proportions to be on a mole basis, that is in terms of # of atoms. When the % mass composition of a sample is known, consider the following five steps 1 assume the masses of the sample exactly 100g Then the example of CH2 O above: 39.99g C, 6.73g H and 53.28g O
- 23. 2 Convert the masses of the elements in the 100.0g sample to amounts in moles. mol C = 39.99g/12.01(g/mol)=3.330mol mol H = 6.73g/1.01(g/mol)=6.66 mol mol O = 53.28g/(16.00g/mol)= 3.330mol 3 Write a tentative formula based on the numbers of mole just determined. C3.330 H6.66 O3.330 4 Attempt to convert the subscript in the tentative formula to small whole #. 5 If the subscripts at this point differ only slightly from whole #, round up them off to whole # to obtain the final formula. If one or more subscripts is not a whole #, multiply all of them by a small whole # that will make all subscript integral. CH2 O C 3.330H 6.66O 3.330 3.330 3.330 3.330
- 24. Combustion Analysis One of the most common methods used to determine empirical formula of a compound. In combustion analysis, a weighted sample of a compound is burned in a stream of oxygen gas. The water vapor and carbon dioxide gas produced in combustion are absorbed by appropriate substance. The increases in mass of these absorbers correspond to the masses of water and carbon dioxide. E.g. Combustion of a sample of a 1.152g of isobutyl propionate, a carbon-hydrogen-oxygen compound, yields 2.726g CO2 and 1.116g H2 O. What is the empirical formula of isobutyl propionate?
- 26. Molecular Formula from Empirical Formula The molecular formula of a compound is a multiple of its empirical formula. For any molecular compound, we can write Moleculas weight = n x empirical formula weight. n = molecular weight____ empirical formula weight Where n is the number of empirical formula units in the molecule.
- 27. E.g. The percentage composition of benzene is 92.3% C and 7.70%H and its molecular weight is 78.12amu. What is the molecular formula of benzene? First, determine the empirical formula from percentage composition
- 28. E.g. A 0.2612g sample of a hydrocarbon produces 0.8661g CO2 and 0.2216g H2 O in combustion analysis. Its molecular mass is found to be 106amu. For this hydrocarbon, determine:(a) its mass percentage composition (b) its empirical formula (c) its molecular formula
- 29. (b) its empirical formula (c) its molecular formula
- 30. IV Oxidation States (O. S.) ~ is related to the number of electrons that an atom loses, gain or otherwise appear to use in joining with other atoms in compounds. ~ is defined to be the charge an atom in a substance would have if the pairs of electrons in each bonded belonged to the more electronegative atom. Whenever two rules appear contradict each other, follow the rule that appears higher on the list. 1 The oxidation state (number) of an individual atom in a free element is 0. E.g the O. S. of a Cl atom in Cl2 or an O atom in O2 is zero. 2 The total of the oxidation states of all the atoms in (a) neutral species, such as isolated atoms, molecules, and formula units, is 0 (b) an ion is equal to the charge on the ion E.g. the sum of the O. S. of all atoms in MnO4 is –1
- 31. 3 In their compounds, the group 1 metals have an O.S. of +1 and the group 2 metals have O.S. of +2. 4 The O.S. of fluorine is –1 in all of its compounds. 5 H has an O.S. 0f +1 in most of its compounds. The exceptions are hydrides, compounds such as NaH in which H is bonded to metallic elements, where the O. S. of H is –1. 6 The usual O. S. of oxygen in a compound is –2. The exceptions are peroxides, such as H2 O2 and Na2 O2 , in which the O. S. of oxygen is –1. 7 In binary compounds with metals, group 17 elements have an O. S. of –1; group 16 elements, 2; and group 15 elements, 3.
- 32. E.g. What is the oxidation state of the underlined element in each of the following? (a) HClO4 (b) MnO4 - (c ) KO2 Nomenclatures- systematic naming of chemical compounds