Summary of PROVE-HF and GUIDE-IT studies by Dr. Vaibhav Yawalkar MD, DM Cardiology
- 1. Prospective Study of Biomarkers, Symptom Improvement and Ventricular Remodeling During Entresto Therapy for Heart Failure (PROVE-HF) James L. Januzzi et al. JAMA September 2, 2019
- 2. Natriuretic Peptides • Five structurally similar peptides – ANP (Primarily produced in Atria) – BNP (Primarily produced in Ventricles) – CNP (Vascular Endothelium) – DNP (dendroaspis natriuretic peptide) – Urodilatin (an isoform of ANP)
- 3. Natriuretic Peptides • Reflect “Myocyte stress” • Higher in HFrEF & Higher NYHA class • Useful biomarkers for HF diagnosis, estimation of HF severity and prognosis – BNP (Breathing Not Properly study) – ProBNP (PRIDE study) • Possible role in management of HF
- 5. Natriuretic Peptides Increased – Age – Renal Failure – Hyperdynamic states – Sepsis Use of ARNI will increase BNP but has no effect on NTproBNP Decreased – Obesity
- 6. Natriuretic Peptides To exclude ADHF • BNP <30-50 pg/ml • NT-proBNP < 300 pg/ml To Rule in ADHF • BNP >100 pg/ml • NT-proBNP >900 pg/ml NT-proBNP, “age-stratified” approach
- 8. Introduction • In the PARADIGM-HF study, sacubitril/valsartan (ARNI) improved outcomes • Remodeling of the myocardium is central to the progression of HFrEF and is associated with risk for cardiovascular events – Effect of ARNI on cardiac remodeling is not known • In PARADIGM-HF, benefits of ARNI were associated with a reduction in NT-proBNP concentrations – Reduction in NT-proBNP during GDMT for HFrEF is associated with reverse cardiac remodeling
- 9. Question • Do changes in NT-proBNP correlate with changes in cardiac structure and function in patients with HFrEF receiving ARNI ?
- 10. Methods • Adult patients with symptomatic HFrEF (LVEF ≤40%) eligible for on- label treatment with ARNI were enrolled • Following discontinuation of ACEI/ARB, ARNI was initiated and titrated • Blood samples were obtained at each study visit for NT-proBNP measurement • An echocardiogram was performed at baseline, 6- and 12-months, and interpreted by a core lab in a clinically and temporally blinded fashion X = Vital status/events (CV death, HF hospitalization, worsening HF), physical examination, blood samples for safety chemistry and biomarkers, urine sampling, HF symptom assessment, KCCQ-23. * Standard HF therapy was continued throughout the study with the exception of ACEI/ARB; †At Day 45, KCCQ-23 was not administered. CV denotes: cardiovascular; HF denotes: heart failure; KCCQ-23 denotes: Kansas City Cardiomyopathy Questionnaire-23. Key Inclusion Criteria Key Exclusion Criteria Aged ≥18 years Patients with HFrEF who are candidates for on-label sacubitril/valsartan treatment per the standard of care NYHA functional class II, III, or IV LVEF ≤40% within the preceding 6 months according to any local measurement, and no subsequent documentation of EF >40% Stable dose of loop diuretic for the 2 weeks preceding study start History of hypersensitivity/allergy or suspected contraindication to ACEI, ARB, or ARNI Any angioedema history Concomitant use of ACEI therapy, nesiritide, aliskiren, or drugs that may affect absorption of the study medication Current or previous treatment with sacubitril/valsartan Inadequate washout of other investigational drugs before study initiation Enrollment in another clinical trial within 30 days of screening Potassium >5.2 mEq/L at screening History of malignancy within 1 year Pregnancy, lactation, or use of any method of contraception that is not highly effective Implantation of CRT/D within 6 months Prior or planned heart transplant or LVAD
- 11. Goals of the study • Primary endpoint: – Correlation between change in NT- proBNP and remodeling at 12 months 1) Left ventricular ejection fraction (LVEF) 2) LV end-diastolic volume index (LVEDVi) 3) LV end-systolic volume index (LVESVi) 4) Left atrial volume index (LAVi) 5) Ratio of early mitral diastolic filling velocity/early diastolic mitral annular velocity (E/e') • Secondary endpoints: – Association between change in NT- proBNP and remodeling at 6 months – Effect of ARNI on cardiac remodeling in specific patient subgroups not represented in the PARADIGM-HF trial: 1) New-onset HF and/or ACEI/ARB naïve 2) Those with BNP or NT-proBNP concentrations below PARADIGM-HF inclusion criteria 3) Patients not reaching target doses of ARNI (97/103 mg twice daily)
- 12. Statistical Method • Pearson correlation coefficients were used to evaluate correlation • Log (NT-proBNP) concentration vs Remodelling Parameters +1 = total positive linear correlation 0 = no linear correlation −1 = total negative linear correlation
- 13. Baseline characteristics (N=794) Parameter N=794 Age, years; mean (SD) 65.1 (12.4) Male sex; n (%) 568 (71.5) NYHA Class II or III; n (%) 780 (98.2) Race; n (%) White Black 581 (73.4) 180 (22.7) Body-mass index, kg/m2, mean (SD) 31.3 (6.9) Past Medical History; n (%) Hypertension Diabetes mellitus Myocardial infarction Atrial fibrillation/flutter 699 (88.0) 361 (45.5) 329 (41.4) 280 (35.3) Guideline-directed HFrEF therapy; n (%) Beta blocker ACEI/ARB MRA CRT/CRT-ICD ICD-alone 757 (95.3) 602 (75.8) 281 (35.4) 122 (15.4) 226 (28.5) Cardiac measurements, median (interquartile range): • LVEF = 28.2 (24.5, 32.7) % • LVEDVi = 86.93 (76.17, 100.43) mL/m2 • LVESVi = 61.68 (51.95, 75.00) mL/m2 • LAVi = 37.76 (31.63, 46.09) mL/m2 • E/e' =11.70 (8.80, 16.00) Subgroups of interest: • New-onset HF and/or ACEI/ARB naïve: N = 118 (14.9%) • BNP or NT-proBNP concentrations below PARADIGM-HF inclusion criteria: N = 292 (36.8%) • Following titration, 278 (35.0%) did not receive target dose
- 14. NT-proBNP concentrations Time point N Median NT-proBNP (25th, 75th percentile), pg/mL Baseline 760 816 (332, 1822) Day 14 754 528 (226, 1378) Day 30 740 546 (211, 1321) Day 45 734 514 (192, 1297) Month 2 721 535 (210, 1299) Month 3 719 488 (211, 1315) Month 6 699 473 (179, 1163) Month 9 659 444 (170, 1153) Month 12 638 455 (153, 1090) Rapid and significant reduction of NT-proBNP was observed, with majority of reduction within the first 2 weeks
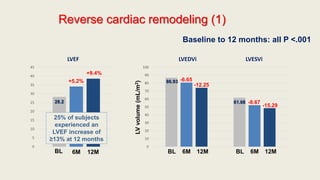
- 15. Primary endpoint • From baseline to 12 months, significant correlations were observed between the change in NT-proBNP concentration and cardiac remodeling parameters. • Analyses demonstrated strong association between early NT-proBNP change and subsequent reverse cardiac remodeling. Parameter Pearson r (IQR) P value NT-proBNP (pg/mL) / LVEF (%) -0.381 (-0.448, -0.310) <.0001 NT-proBNP (pg/mL) / LVEDVi (mL/m2) 0.320 (0.246, 0.391) <.0001 NT-proBNP (pg/mL) / LVESVi (mL/m2) 0.405 (0.335, 0.470) <.0001 NT-proBNP (pg/mL) / LAVi (mL/m2) 0.263 (0.186, 0.338) <.0001 NT-proBNP (pg/mL) / E/E’ 0.269 (0.182, 0.353) <.0001
- 16. Reverse cardiac remodeling (1) 0 5 10 15 20 25 30 35 40 45 LVEF 0 10 20 30 40 50 60 70 80 90 100 LVEDVi LVESVi BL 6M 12M LVvolume(mL/m2) +5.2% +9.4% -6.65 -12.25 -8.67 -15.29 BL 6M 12M BL 6M 12M Baseline to 12 months: all P <.001 28.2 86.93 61.68 25% of subjects experienced an LVEF increase of ≥13% at 12 months
- 17. 0 5 10 15 20 25 30 35 40 LAVi Reverse cardiac remodeling (2) 0 2 4 6 8 10 12 14 E/e’ BL 6M 12M BL 6M 12M -4.36 -7.57 -1.23 -1.30 E/e’ratio 37.76 11.70 LVMi fell from 124.77 to 107.82 g/m2 (mean -16.00 g/m2; P <.001) Baseline to 12 months: all P <.001
- 18. Subgroups of interest • Reverse cardiac remodeling was comparable in each subgroup of interest New-onset HF/ACEI-ARB naïve (N=118) Parameter LS Mean change, BL to 12 months (95% CI) LVEF (%) +12.8 (+11.05, +14.5) LVEDVi (mL/m2) -13.81 (-15.78, -11.83) LVESVi (mL/m2) -17.88 (-20.07, -15.68) LAVi (mL/m2) -8.44 (-9.73, -7.15) E/e’ -2.60 (-3.83, -1.37) NP < PARADIGM incl criteria* (N=292) Parameter LS Mean change, BL to 12 months (95% CI) LVEF (%) +9.4 (+8.6, +10.3) LVEDVi (mL/m2) -11.32 (-12.24, -10.40) LVESVi (mL/m2) -14.15 (-15.15, -13.15) LAVi (mL/m2) -7.06 (-7.54, -6.58) E/e’ -0.93 (-1.43, -0.43) Not reaching target dose (N=278) Parameter LS Mean change, BL to 12 months (95% CI) LVEF (%) +9.4 (+8.4, +10.3) LVEDVi (mL/m2) -10.99 (-12.21, -9.77) LVESVi (mL/m2) -14.32 (-15.67, -12.97) LAVi (mL/m2) -7.23 (-7.97, -6.50) E/e’ -0.46 (-1.32, +0.40); P =NS All P <0.001 except where noted
- 19. Death or HF hospitalization by 12 months Patients with largest reduction in NT-proBNP and LVESVi by 6 months had lowest rates of subsequent death or HF hospitalization by 12 months
- 20. Adverse events by 12 months • Adverse events rates were similar to the PARADIGM-HF study, with the exception of dizziness • Positively-adjudicated angioedema occurred in only 2 patients (0.3%), of whom 1 was Black (0.56%) • Both cases of angioedema were mild, resolving with antihistamines or no therapy Event N = 794; n (%) Hypotension (systolic blood pressure <90 mm mercury) 140 (17.6) Dizziness 133 (16.8) Hyperkalemia (potassium > 5.3 milliequivalents/liter) 105 (13.2) Worsening kidney function* 98 (12.3) Angioedema No treatment or antihistamines only without hospitalization 2 (0.3) *Worsening (decrease) in estimated glomerular filtration rate of ≥ 35% from baseline, or an increase in creatinine of ≥ 0.5 mg/dL from baseline and a worsening (decrease) in estimated glomerular filtration rate of ≥ 25% from baseline at a given visit.
- 21. Limitations • Single-group, open-label design – Availability of ARNI & its Class I guideline recommendation made a comparison group unethical for a 12-month study – Echocardiograms were interpreted in a temporally-blinded manner and primary endpoint was based on objective measures • Multiple comparisons may have increased risk of Type 1 error • Not all echo measurements were available at each time point
- 22. Conclusions • In this study of patients with HFrEF treated with sacubitril/valsartan, reduction in NT-proBNP was significantly associated with reverse cardiac remodeling • The degree of reverse remodeling demonstrated may help to explain how sacubitril/valsartan reduces morbidity and mortality in patients with HFrEF • Analyses examining impact of sacubitril/valsartan on quality of life, symptoms, and a broad range of mechanistic biomarkers are underway
- 23. Effect of Natriuretic Peptide–Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With HFrEF GUIDE-IT Study
- 24. Introduction • NTproBNP levels decline in response to GDMT in HFrEF • Rising levels are associated with poor prognosis. • Serial measurements of natriuretic peptides may be used to guide titration of long-term medical therapy in HF • Hypothesis not tested in large & powered trials
- 25. Question Does a strategy of titrating therapy to a specific NT- proBNP target improve clinical outcomes in high-risk patients with HFrEF ?
- 26. GUIDE-IT Study • Patients with HFrEF (EF<40%) randomized to either an NT- proBNP–guided strategy or usual care. • HF therapy titrated with the goal of achieving a target NT- proBNP of less than 1000 pg/mL • Primary end point: – composite of time-to-first HF hospitalization or cardiovascular mortality
- 27. Inclusion Criteria • HFrEF, EF < 40% • HF hospitalization in prior 12 months • NTproBNP > 2000 pg/ml • BNP > 400 pg/ml • ACS or Revascularization in last 30 days • CRT within 3 months • End stage Renal disease • Anticipated heart transplantation in next 12 months Exclusion Criteria
- 28. Methods • Patients were randomized in a 1:1 fashion • In NT-proBNP-guided strategy, clinicians were instructed to titrate HF therapy to target an NT-proBNP level of less than 1000 pg/mL. • Dosage particularly of neuro-harmonal antagonists were titrated • Planned Follow up after 2 weeks , 6 weeks & then every 3 months for 24 months. (Unplanned follow up for HF event)
- 29. GUIDE-IT Study • The study met prespecified inefficacy criteria and further enrollment was stopped. (894 out of target 1100 enrolled) • No difference in outcomes in two groups. • Other 11 trials had shown a reduction in all-cause mortality with natriuretic peptide-guided therapy compared with usual care • In this study: Up-titration of therapy in NTproBNP group was lesser Target dosage of GDMT were not achieved Patients had advanced HF compared to other trials
- 32. GUIDE-IT Study Conclusion In high-risk patients with HFrEF, a strategy of NT-proBNP guided therapy was not more effective than a usual care strategy in improving outcomes.
- 33. THANK YOU